7.3.5—
Cell Motility; Cilia and Flagella
Microtubules, arranged in a characteristic 9+2 configuration (Fig. 7.8a), are the major structural components of flagella, cilia and sperm tails (Warner, 1974). The flagellar apparatus consists of a membrane-bounded, slender, cylindrical cell extension subtended by its basal body, the intracellular organelle which appears to be its origin and kinetic centre.
Basal bodies are identical in structure and apparently homologous with the centrioles of animal and lower plants cells. During mitosis, centrioles appear to determine the poles of the spindle axis and to organize the assembly and alignment of spindle microtubules. Following mitosis, in flagellated cells, the centrioles migrate to the cell periphery and organise the assembly of the flagellar apparatus. The mechanism whereby centrioles act as microtubule initiating centres, the reasons for their stability during the life cycle of the cell, and the factors controlling basal body replication in multiflagellated cells, are all unknown.
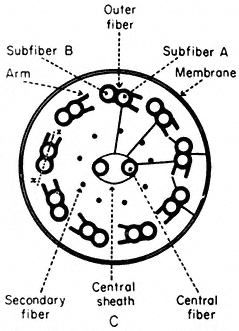
Figure 7.8a
Diagram of flagella cross section, showing
arrangement of microtubules, arms and cross-links.
Basal bodies are composed of nine sets of triplet microtubules (A, B and C) with lateral connections between the A and C tubules of adjacent triplets. At the proximal end of each basal body, a thin filament runs from the A tubule of each triplet to a central hub, forming a cartwheel pattern. At the distal end is the complex transitional region between the microtubules of the basal body and those of the cilium.
The shaft of the cilium is characterized by a central pair of axially oriented microtubules, surrounded by a ring of nine doublets (Fig. 7.8a). The A sub-fibre of a doublet possesses a complete wall of 13 protofilaments. However, the B subfibre has only 10 protofilaments in its wall, and shares the three protofilaments of the A tubule forming the common partition between them.
From the A tubule of a doublet, two short arms project towards the B tubule of the adjacent doublet. These arms have been isolated and shown to possess Mg2+ -dependent ATPase activity (Gibbons, 1965). The arms are approximately 30 nm long, 9 nm wide, and spaced at intervals of 16–22 nm along the length of the A tubule. The enzyme has been called dynein after its postulated role in converting chemical energy into mechanical force. In addition to the dynein arms, cross-bridges have been seen extending between the outer doublets and the flagellar membrane. Radial links, connecting the central pair of tubules with the outer doublets, have also been described. Further, the two microtubules of the central pair differ from one another, one member exhibits two rows of short projections, 18 nm long, and spaced at intervals of 16 nm. Thus, a complex of radially or longitudinally arranged arms, bridges or filaments is present in the flagellar matrix in association with microtubules (Fig. 7.8b).
The most widely accepted hypothesis to account for flagellar motion is the sliding microtubule model (Satir, 1974). Applied to flagella, the hypothesis states that the force responsible for motion is produced when the axonemal
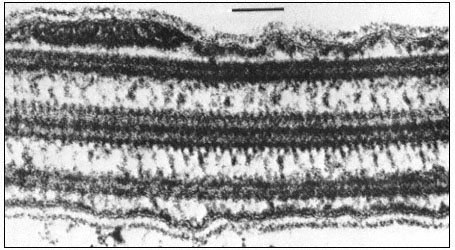
Figure 7.8b
Longitudinal section of a flagellum showing connections between the central and
outer doublet microtubules, and bridges between the peripheral tubules and the cell
membrane. Scale marker 0.1 µm. Fig. 8.8b. from Ringo (1967 J. Cell Biol. 33, 543–71,
reproduced by permission of The Rockefeller University Press.
microtubules, which do not change in length, tend to slide with respect to one another. Accordingly, the model predicts that in different stroke positions, the morphological relationships of the microtubules will change in a systematic fashion, so that the geometry of the bent flagellum will be reflected in the displacement of the microtubules. This has, indeed, been demonstrated using serial sections of the lateral gill cilia of the freshwater mussel.
Other evidence has also accumulated to support this hypothesis. Glycerinated or demembranated flagella beat normally upon the addition of ATP and Mg2+ or Ca2+ ions. When such naked axonemes are briefly treated with trypsin, the circumferential and radial links holding the axoneme together are interrupted, whereas the dynein arms are trypsin-resistant. Addition of ATP now causes a sliding of the microtubules, so that the axoneme grows very much longer and thinner as groups of doublets crawl over one another. This sliding must normally be converted to bending by the series of intermicrotubule connections, within the axoneme.
Thus, microtubules are implicated, in both plant and animal cells, in a variety of processes involving the active movement of cells or cellular components. However, the possibility that a single basic mechanism of action underlies their seemingly diverse roles is still in doubt. The dynamic equilibrium model involving assembly and disassembly of microtubules cannot be applied to the stable microtubules of flagella or the cytoplasmic microtubules of higher plant cells. Neither the assembly-disassembly hypothesis, nor the sliding filament model alone appears adequate to explain the complex functioning of
microtubules in the mitotic apparatus. Even within a process such as spindle elongation, microtubules may function in different ways. For example, the spindles of HeLa cells show an initial rapid phase of elongation, followed by a subsequent slow phase: colchicine only inhibits the slow phase (presumably by interfering with microtubule assembly) but does not affect the rapid phase which may be based on a sliding mechanism. Furthermore, dividing plant and animal cells are remarkably different in their sensitivity to drugs: the spindles of many plant cells require treatment with approximately 1,000-fold higher concentrations of a wide variety of anti-mitotic drugs to achieve mitotic arrest.