7.3—
Biological Studies
Microtubules appear to be involved in several functional roles within the plant cell (see Newcomb, 1969). For convenience, these can be grouped under five headings: (1) Cytoskeletal role; generation and maintenance of cell shape (2) Cell wall architecture; cell differentiation (3) Intracellular transport (4) Chromosome movements; cell plate formation (5) Cell motility; cilia and flagella. In this section, we shall consider the involvement of microtubules in these phenomena, with emphasis on possible operative mechanisms, and drawing, and drawing, where necessary, on information obtained from studies of animal cells.
7.3.1–
Cytoskeletal Role
Despite the forces of surface tension, animal and some lower plant cells can maintain a non-spherical shape in the absence of a rigid cell wall. Numerous electron microscope studies have revealed that microtubules are present in large numbers and with distinctive orientations in such asymmetric cells or cell extentions. Correlations between cell asymmetry and microtubules have been extensively studied in animal cells, for example in erythrocytes, explanted nerve ganglia or neuroblastoma cells, lens epithelial cells and mesenchyme cells of echinoderm embryos. When these cells are treated with microtubule depolymerizing agents (high hydrostatic pressure, low temperature or colchicine), they assume spherical shapes.
The unicellular alga, Ochromonas, lacks a cell wall or pellicle, yet has a characteristic pear-shape, with a narrow tail or rhizoplast, and a bulbous anterior bearing a small projection, the beak. Two sets of microtubules are involved in maintaining this shape. The plasma membrane of the main cell body is underlain by a series of curved microtubules which extend into the rhizoplast. The other set of microtubules is associated with the beak complex. Disassembly of the microtubules with colchicine or hydrostatic pressure leads to loss of the characteristic cell shape; removal of the depolymerizing agent allows the normal shape to regenerate even in the absence of protein synthesis (Brown & Bouck, 1973).
The two sets of microtubules in Ochromonas are differentially sensitive to colchicine and pressure. At relatively high concentrations of colchicine (10 mg ml–1 ) or at high pressure (8,000 psi), both sets of microtubules are affected but at different rates: first, disassembly of rhizoplast tubules is correlated with disappearance of the posterior tail; this is followed by loss of beak tubules and beak asymmetry. Lower concentrations of drug (5 mg ml–1 ) or lower pressure (6,000 psi) selectively disassemble only the rhizoplast microtubules. These results possibly indicate separate roles for each set of microtubules in determining the shape of this cell. Assuming that different tubulins are not involved, the beak and rhizoplast microtubules may represent two equilibrium systems competing for a common pool of subunits. Alternatively, the differential sensitivity of the two systems, and their sequential reassembly, may reflect characteristics of the sites which initiate microtubule polymerization.
The protozoan, Actinosphaerium, has been extensively used in similar studies on the morphopoietic function of microtubules. The organism is a spherical cell bearing numerous, long, relatively stiff, protoplasmic extensions or axopods, containing a central core, the axoneme (Fig. 7.3a). Each axoneme is composed
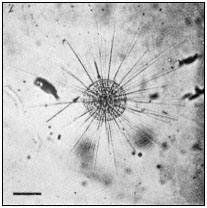
Figure 7.3a
Photomicrograph of Actinosphaerium
showing the slender cell extensions
or axopods radiating from the cell
body. Scale marker 100 µ m.
of several hundred axially aligned microtubules, arranged into two rows that coil about a central axis (Fig. 7.3b). This strict pattern does not seem to be determined by a template of initiating sites, since, during axopod growth, the microtubule array is initially disorganized and becomes progressively more geometrically perfect.

Figure 7.3b
Cross section through an axoneme of Actinosphaerium
near the axopod base. Two spiral rows of microtubules are
coiled about a central axis in a precise geometrical arrangement.
Scale marker 1 µm. From Tilney & Porter (1965) Protoplasma 60,
317–43, reproduced by permission of the authors and Springer-Verlag.
When low temperature, pressure or drugs are employed to depolymerize microtubules of Actinosphaerium, the axopods undergo immediate retraction. In contrast, D2 O, which stabilizes microtubules, prevents the breakdown of axopods by cold or pressure. Retractions and extensions are a normal feature of individual axopods and serve to move the cell across its substratum. Thus, the cell must exert a fine control over the behaviour of microtubules in each axopod separately (see Gunning & Steer, 1975).
In flowering plants, the male sperm is formed from the generative cell which usually has no cell wall and lies within the pollen grain. Growth of the pollen tube is accomplished by the vegetative cell, while the generative cell adopts a spindle-shape before entering and travelling down the pollen tube. Development of the spindle shape is accompanied by the appearance of microtubules aligned parallel to the long axis of the generative cell. Treatment of the generative cell with drugs leads to a loss of microtubules and the cell adopts a spherical form (Sanger & Jackson, 1971).
Thus, microtubules are apparently involved in both generating and maintaining changes in cell shape. The mechanisms regulating orientation and assembly of microtubules in this role are still unknown. Microtubules in the
axonemal cores of Actinosphaerium axopods are connected with their neighbours by cross-bridges. Whether these lateral links function in stabilizing the extended structure or in orienting the microtubules has yet to be determined.
7.3.2—
Cell Wall Architecture
During interphase in higher plant cells, cytoplasmic microtubules lie close to the cell membrane and are arranged circumferentially along the lateral walls of the cells but are randomly disposed underlying the transverse walls (Fig. 7.5a). In the earliest paper reporting the presence of microtubules in higher plant cells, Ledbetter and Porter (1963) noted the parallel alignment of cellulosic microfibrils of the wall and the subjacent cytoplasmic microtubules (Fig. 7.4). Numerous subsequent studies have confirmed this observation, in algal cells and in higher plant cells undergoing both primary and secondary wall formation (see Hepler & Palevitz, 1974).
In the long xylem fibres of Salix, wall microfibrils are deposited in two different orientations simultaneously in the middle and at the extremities of the cell. Even in this situation, microtubule alignment mirrors that of the overlying microfibrils, indicating the ability of the cell to maintain two sets of microtubule-microfibril associations. In the lorica stalk of the alga, Poteriochromonas, the primary wall microfibrils are arranged helically and tend to fasciate into ribbonlike fibrils, 20 nm in width. Every such wall fibril coincides precisely with a microtubule, the plasmalemma separating the two sets of linear structures (Schnepf et al., 1975).
Other examples of the association between microtubules and microfibrils are afforded by cells undergoing irregular or sculptured deposition of secondary wall material. During xylogenesis, microtubules are specifically grouped under the developing wall thickenings in vessels, and, once again, are oriented parallel to the microfibrils. Similar situations prevail during the development of wall thickenings in differentiating guard cells and during the deposition of the nacreous walls of sieve elements.
Attempts have been made to confirm the association between wall microfibrils and microtubules by treating cells with colchicine. However, plant tissues seem relatively resistant to a wide variety of drugs (Heath, 1975b) and, even at high concentrations of colchicine, not all cytoplasmic microtubules in plant cells are depolymerized (Pickett-Heaps, 1967; Wooding, 1969). Also, such levels of colchicine are known to affect other cellular processes, in particular, membrane-related phenomena (see Wilson & Bryan, 1974). Therefore, although wall microfibril orientation is often distorted in the presence of colchicine, such results should be interpreted with caution.
7.3.3—
Intracellular Transport
There is much evidence to indicate that wall precursors are transported to the wall in dictyosome-derived vesicles. On the other hand, there is no evidence to
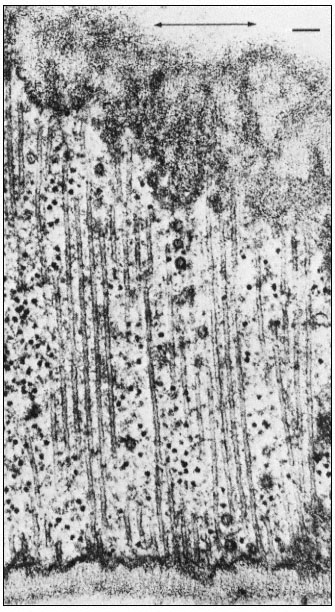
Figure 7.4
Microtubules running parallel to one another immediately beneath
a primary cell wall in root tips of bean ( Phaseolus vulgaris ). The
microfibrils of the wall are oriented parallel to the microtubules. The
axis of cell elongation (indicated by the arrow) is at right angles to the
orientation of the microtubules and microfibrils. Vesicles can be seen
among the microtubules. Scale marker 0.1 µ m. From Newcomb (1969)
A. Rev. Pl. Physiol. 20, 253–88, reproduced by permission
of the author and Annual Reviews, Inc.
suggest that microtubules provide the motive force for such transport. However, it has been proposed that the parallel microtubules girdling the cells of higher plants peripherally provide a framework for the guidance and alignment of these vesicles. In some cases the microtubules lie so close to one another that it has been argued that they could function only by excluding vesicles or elements of the endoplasmic reticulum, preventing their fusion with the plasmalemma at certain sites. In either case, a vectorial role for microtubules is indicated.
Ledbetter and Porter suggested that microtubules may be involved in orienting and even driving cyclosis of cytoplasm, a process which might indirectly be responsible for the alignment of wall microfibrils. However, several lines of evidence have recently implicated cytoplasmic microfilaments in this role (Hepler & Palevitz, 1974). These filaments, 5–8 nm in width, are more consistently located and aligned within the zones of streaming cytoplasm. The microfilaments are morphologically identical to F-actin, one of the major contractile proteins of muscle. Recently, microfilaments in cells of both Nitella and higher plants have been decorated with heavy meromyosin, a characteristic test for actin-like filaments. In primitive organisms, such as the slime mould Physarum, there is overwhelming evidence to indicate that similar micro-filaments provide the motive force for rapid cytoplasmic streaming.
However, around the axonemes of Heliozoan axopods described earlier, cytoplasmic particles stream to and from the cell body. Microfilaments have not been seen in this structure. By contrast, in the giant coenocytic alga, Caulerpa, microtubules are located within the zones of, and parallel to, the numerous sluggish cytoplasmic streams. Mitochondria, plastids and other cytoplasmic components are constantly circulated to and from the growing tips of this large, asymmetric cell. In the chromatophores of animal cells, microtubules have been shown to be essential to the movements of pigment granules. Anti-mitotic agents depolymerize microtubules and simultaneously disrupt the alignment and arrest the movement of the pigment granules (Murphy, 1975). Thus, it remains possible that certain types of cytoplasmic transport require a microtubule framework in both a vectorial and an active role.
7.3.4—
Cell Division
At least two types of microtubule can be distinguished in most spindles, those that traverse the spindle from pole to pole (continuous fibres), and those that connect the kinetochores of the chromosomes to the poles (chromosomal or kinetochore fibres). During separation of chromatids in anaphase, the continuous fibres lengthen, increasing the distance between the poles, while the chromosomal fibres usually—but not always—shorten, further separating the chromatids. After telophase, the continuous spindle may persist and be added to in the interzonal region by numerous additional microtubules, leading to formation of the phragmoplast and eventually, the cell plate.
Considerable changes in microtubule organization (Fig. 7.5, A–F) take
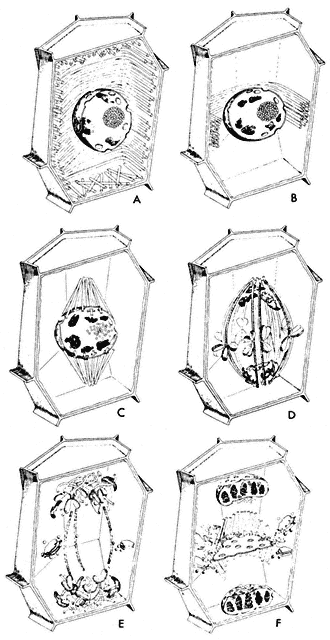
Figure 7.5
Diagram of changes in microtubule orientation and distribution during the phases
of mitosis in a higher plant cell. A—interphase; B—preprophase; C—prophase;
D—metaphase; E—anaphase; F—telophase. From Ledbetter & Porter (1970)
Introduction to the Fine Structure of Plant Cells, p. 44, Springer-Verlag, Berlin,
reproduced by permission of the authors and Springer-Verlag.
place during the events of a typical mitotic cycle (Fuge, 1974). With the onset of division, the peripheral cytoplasmic microtubules of the interphase cell disappear. Concomitantly, a band of microtubules is formed, several layers deep, lying close to the cell wall and girdling the nucleus perpendicularly to the prospective spindle axis. This preprophase band of microtubules appears to predict the plane of the future cell plate (Pickett-Heaps & Northcote, 1966). This has been demonstrated in divisions destined to give daughter cells of different size, for example in the asymmetric divisions of guard mother cells of wheat.
At the beginning of prophase, the number of microtubules in the preprophase band decreases, and new microtubules appear close to the nuclear membrane, this time running parallel to the prospective spindle axis. The microtubules in this so-called 'clear zone' in turn appear to furnish protein material for the spindle. It is possible that a total transformation of these extranuclear microtubules into spindle microtubules takes place.
Prior to spindle formation, the nuclear envelope either disintegrates or becomes perforated at the prospective poles, and cytoplasmic (clear zone) microtubules enter the karyoplasm at these sites. Some of these microtubules appear to establish connections with the chromosomal kinetochores. Since the number of kinetochore microtubules (kMts) increases as prometaphase proceeds, and since clear zone microtubules can be utilized only immediately after breakdown of the nuclear envelope, it is likely that de novo microtubule assembly also occurs at the kinetochores. Thus, it appears that the kinetochore, which varies in appearance in different organisms from amorphous to highly structured, acts as an initiating site or microtubule morganizing centre (MTOC).
In prometaphase, continuous fibres also begin to form and to increase in number. There is some controversy as to whether in higher organisms the continuous microtubules pass from pole to pole. A large proportion appear to overlap in the interzone between the half-spindles (Fig. 7.6), and have been termed non-kinetochore microtubules (nkMts). In lower plants, and all animal cells, with centrioles, the continuous or nkMts originate near the centrioles. These loci are represented by amorphous, electron-dense aggregates of material, and behave as another set of MTOCs. The essential feature of the centriole in mitosis seems to be in determining the spindle axis. In higher plants that lack centrioles, neither the location nor form of polar MTOCs is clear.
During metaphase, engagement of sister chromatids to opposite poles takes place via microtubules, followed by movement of the chromatids to the equatorial plate. At this stage, the maximum number of spindle microtubules is attained, the numbers often trebling between prometaphase and metaphase. Estimates from microtubule counts in serial sections of the metaphase spindle of the sea urchin, Arbacia, indicate a total microtubule length of 50,000 µm. The very large
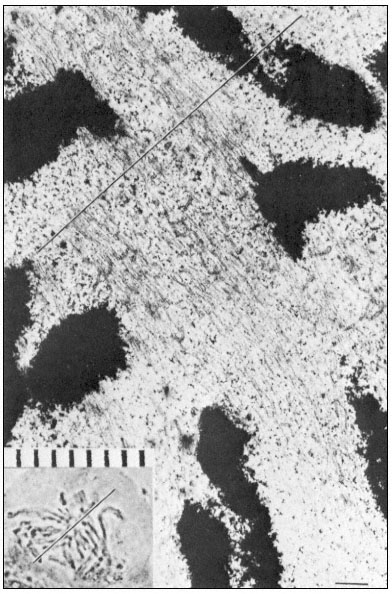
Figure 7.6
Electron and light micrographs of a dividing cell in prometaphase showing
kinetochore and nonkinetochore microtubules. The line indicates the equatorial
plate. Scale marker 1 µm. From Bajer (1968) Symp. Soc. exp. Biol. 22, 285–310,
reproduced by permission of the author and University Press, Cambridge.
spindles of Haemanthus endosperm cells contain considerably more microtubules than those of Arbacia.
Anaphase separation of the chromatids involves direct translation of the kinetochores at almost uniform velocity (0.2–4 µm min–1 ) towards their respective poles. Two experimentally separable processes are involved: first, a movement of the chromatids towards their poles, during which the chromosomal fibres shorten to as little as 20% of their metaphase length; secondly, an elongation of the spindle itself, during which the distance between the poles increases, thereby further separating the two groups of chromatids.
Time-lapse films of dividing Haemanthus endosperm cells show that many other objects, such as vesicles and granules, are commonly transported polewards during prometaphase-anaphase. Conversely, in onion root tip cells, the poleward movement of the chromosomes is accompanied by a reciprocal movement of other materials from the poles towards the mid-plate. Elements of the endoplasmic reticulum accumulate at the poles during metaphase, enter the spindle at anaphase, pass between the chromosomes moving in the opposite direction, and eventually aggregate and coalesce at the mid-plate to give rise to the transverse cell wall at telophase.
Dissolution of kMts and nkMts during telophase is accompanied by the formation of new microtubules in the interzone, leading to the formation of the phragmoplast in higher plant cells (Fig. 7.7). Phragmoplast microtubules appear to be involved in the movement, alignment and fusion of ER- and
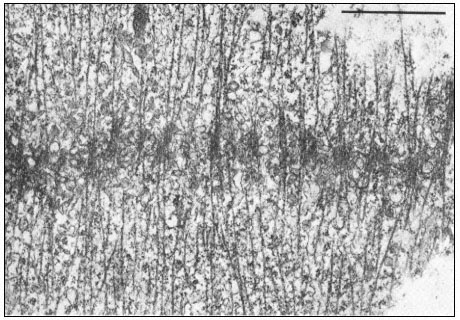
Figure 7.7
Phragmoplast microtubules at the mid-plate during telophase.
Vesicles contributing to the forming cell plate can be seen aligned among the
microtubules. Scale marker 1 µm. From Hepler & Jackson (1974) J. Cell. Biol. 38, 437–46,
reproduced by permission of the authors and The Rockefeller University Press.
dictyosome-derived vesicles to form the cell plate—yet another example of microtubule-associated intracellular transport.
Thus, distinct changes in the polarity and distribution of spindle microtubules take place during the various phases of the mitotic cycle. It should be borne strongly in mind that such changes occur under conditions of very low protein synthesis. Consequently, both assembly and functioning of the mitotic apparatus must involve regulation of microtubule polymerization at a physico-chemical level. Interaction of microtubules with other components of the mitotic apparatus must also be regulated at this level. Several hypotheses have been proposed to explain the mechanism of mitotic movements, although little direct experimental evidence to support the models has yet been obtained.
One prevailing hypothesis to account for spindle elongation (Brinkley & Cartwright, 1971) is that the poles are pushed apart by tip growth of continuous microtubules in the course of which subunits from disassembled kMTs may be utilized. Shortening of the chromosomal fibres is thought to occur by depolymerization at the poles.
Another hypothesis based on the sliding of adjacent spindle microtubules was proposed by Mcintosh et al., (1969). Earlier, several workers had reported the existence of arms or cross-bridges between spindle microtubules. The basis of the sliding tubule hypothesis is that the intertubular bridges in the spindle serve as mechano-chemical elements. Sliding of two microtubules against each other is thought to be generated by the successive breaking and reforming of cross-bridge linkages, a process that is energy-consuming and mediated via ATPase activity of the bridges. It has been estimated that the energy released through the hydrolysis of approximately 20 ATP molecules would suffice to move an average chromosome from the metaphase plate to the pole.
A prerequisite for the McIntosh model is an ordered system of long microtubules, emanating from the poles and reaching far into the opposite half-spindles in metaphase. Microtubules from opposite poles are suggested to have antiparallel polarity with regard to the direction in which their lateral arms could produce force. The kMts in each half-spindle would also show polarity; their lateral arms would only produce force directed towards the spindle equator. If one of the long interpolar microtubules and a kMt of antiparallel polarity came into contact at anaphase, the two tubules, due to the directed activity of their arms, would push themselves in opposite directions by sliding. The model is analogous to the generally accepted model for the interaction of actin and myosin in skeletal muscle. The mechanism could cause chromosome separation, as well as spindle elongation when anti-parallel nkMts interact where they overlap.
A third hypothesis involving actin-microtubule interactions (Forer & Behnke, 1972) has derived from observations of actin-like thin filaments in the mitotic or meiotic spindles of both animal and plant cells. Fluorescein-labelled heavy meromyosin binds to isolated sea urchin spindles. At the ultrastructural level, microfilaments within the spindle have been decorated with heavy
meromyosin, a test for actin-like proteins (Gawadi, 1971; Forer & Behnke, 1972). Several other recent publications have confirmed the presence of actinlike filaments in dividing cells. In kangaroo rat cells, actin was only demonstrable in association with chromosomal fibres (Sanger, 1975), suggesting an actinmyosin type interaction as the force-producing mechanism for chromosome movements, that is, for the autonomous movements of chromosomes in anaphase, as opposed to spindle elongation which may be controlled solely by microtubule assembly-disassembly.
Myosin-like proteins have not yet been located in spindles. Several workers have suggested that microtubules might serve as rigid structures against which the actin filaments could exert a contractile force by the formation and breakage of cross-links. A Ca2+ -dependent ATPase (similar in this respect to both myosin and dynein) is present in the isolated spindle in a concentration three times that in the cytoplasm (Mazia et al., 1972).
The theories on the mechanisms involved in chromosome separation have arisen from observations of living or fixed, intact cells. Such studies are hampered by the inability to experiment directly with the mitotic apparatus, except as it exists in the complexity of its cellular environment. Exciting possibilities have been raised by recent successful attempts to isolate and experiment with the mitotic apparatus in vitro. Intact spindles from the eggs of the surf clam show normal sensitivity to temperature and colchicine. These isolated spindles can incorporate brain tubulin during their reassembly and such hybrid spindles are also cold labile, Ca2+ -sensitive and capable of considerable increase in overall length (Rebhun et al., 1974). Moreover, early anaphase spindles isolated from kangaroo rat cells will continue chromosome motion, in the absence of exogenous spindle subunits, when ATP is added (Cande et al., 1974). These results already suggest that while spindle growth requires microtubule polymerization, anaphase movements do not.
7.3.5—
Cell Motility; Cilia and Flagella
Microtubules, arranged in a characteristic 9+2 configuration (Fig. 7.8a), are the major structural components of flagella, cilia and sperm tails (Warner, 1974). The flagellar apparatus consists of a membrane-bounded, slender, cylindrical cell extension subtended by its basal body, the intracellular organelle which appears to be its origin and kinetic centre.
Basal bodies are identical in structure and apparently homologous with the centrioles of animal and lower plants cells. During mitosis, centrioles appear to determine the poles of the spindle axis and to organize the assembly and alignment of spindle microtubules. Following mitosis, in flagellated cells, the centrioles migrate to the cell periphery and organise the assembly of the flagellar apparatus. The mechanism whereby centrioles act as microtubule initiating centres, the reasons for their stability during the life cycle of the cell, and the factors controlling basal body replication in multiflagellated cells, are all unknown.
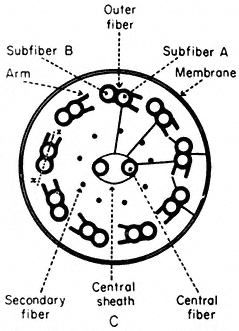
Figure 7.8a
Diagram of flagella cross section, showing
arrangement of microtubules, arms and cross-links.
Basal bodies are composed of nine sets of triplet microtubules (A, B and C) with lateral connections between the A and C tubules of adjacent triplets. At the proximal end of each basal body, a thin filament runs from the A tubule of each triplet to a central hub, forming a cartwheel pattern. At the distal end is the complex transitional region between the microtubules of the basal body and those of the cilium.
The shaft of the cilium is characterized by a central pair of axially oriented microtubules, surrounded by a ring of nine doublets (Fig. 7.8a). The A sub-fibre of a doublet possesses a complete wall of 13 protofilaments. However, the B subfibre has only 10 protofilaments in its wall, and shares the three protofilaments of the A tubule forming the common partition between them.
From the A tubule of a doublet, two short arms project towards the B tubule of the adjacent doublet. These arms have been isolated and shown to possess Mg2+ -dependent ATPase activity (Gibbons, 1965). The arms are approximately 30 nm long, 9 nm wide, and spaced at intervals of 16–22 nm along the length of the A tubule. The enzyme has been called dynein after its postulated role in converting chemical energy into mechanical force. In addition to the dynein arms, cross-bridges have been seen extending between the outer doublets and the flagellar membrane. Radial links, connecting the central pair of tubules with the outer doublets, have also been described. Further, the two microtubules of the central pair differ from one another, one member exhibits two rows of short projections, 18 nm long, and spaced at intervals of 16 nm. Thus, a complex of radially or longitudinally arranged arms, bridges or filaments is present in the flagellar matrix in association with microtubules (Fig. 7.8b).
The most widely accepted hypothesis to account for flagellar motion is the sliding microtubule model (Satir, 1974). Applied to flagella, the hypothesis states that the force responsible for motion is produced when the axonemal
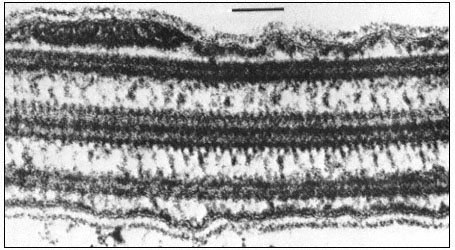
Figure 7.8b
Longitudinal section of a flagellum showing connections between the central and
outer doublet microtubules, and bridges between the peripheral tubules and the cell
membrane. Scale marker 0.1 µm. Fig. 8.8b. from Ringo (1967 J. Cell Biol. 33, 543–71,
reproduced by permission of The Rockefeller University Press.
microtubules, which do not change in length, tend to slide with respect to one another. Accordingly, the model predicts that in different stroke positions, the morphological relationships of the microtubules will change in a systematic fashion, so that the geometry of the bent flagellum will be reflected in the displacement of the microtubules. This has, indeed, been demonstrated using serial sections of the lateral gill cilia of the freshwater mussel.
Other evidence has also accumulated to support this hypothesis. Glycerinated or demembranated flagella beat normally upon the addition of ATP and Mg2+ or Ca2+ ions. When such naked axonemes are briefly treated with trypsin, the circumferential and radial links holding the axoneme together are interrupted, whereas the dynein arms are trypsin-resistant. Addition of ATP now causes a sliding of the microtubules, so that the axoneme grows very much longer and thinner as groups of doublets crawl over one another. This sliding must normally be converted to bending by the series of intermicrotubule connections, within the axoneme.
Thus, microtubules are implicated, in both plant and animal cells, in a variety of processes involving the active movement of cells or cellular components. However, the possibility that a single basic mechanism of action underlies their seemingly diverse roles is still in doubt. The dynamic equilibrium model involving assembly and disassembly of microtubules cannot be applied to the stable microtubules of flagella or the cytoplasmic microtubules of higher plant cells. Neither the assembly-disassembly hypothesis, nor the sliding filament model alone appears adequate to explain the complex functioning of
microtubules in the mitotic apparatus. Even within a process such as spindle elongation, microtubules may function in different ways. For example, the spindles of HeLa cells show an initial rapid phase of elongation, followed by a subsequent slow phase: colchicine only inhibits the slow phase (presumably by interfering with microtubule assembly) but does not affect the rapid phase which may be based on a sliding mechanism. Furthermore, dividing plant and animal cells are remarkably different in their sensitivity to drugs: the spindles of many plant cells require treatment with approximately 1,000-fold higher concentrations of a wide variety of anti-mitotic drugs to achieve mitotic arrest.