Seven—
Juvenile Survivorship of Northern Elephant Seals
Burney J. Le Boeuf, Patricia Morris, and Joanne Reiter
ABSTRACT. The aim of this study was to determine the juvenile survivorship rate of northern elephant seals, Mirounga angustirostris , throughout the first four years of life and to assess the role of year, cohort, and condition at weaning on survival.
The study was conducted at Año Nuevo, California, during the years 1971–1978, a time when colony size was increasing. Pup mortality on the rookery prior to weaning was estimated from daily censuses during the breeding season. Juvenile survivorship was determined from resights of 8,362 individuals tagged on the rookery at weaning (about 30 days of age); systematic searches were conducted on the natal rookery as well as on neighboring rookeries. The effect of mass and length at weaning on juvenile survivorship to 1 and 2 years of age was determined from 734 weaned pups weighed and measured during the years 1978 and 1984–1988.
Mean percentage survival to age 1 was 36.8 ± 8.5; to age 2, 26.3 ± 6.3; to age 3, 19.4 ± 5.1; and to age 4, 16.3 ± 5.2. Most of the first-year mortality occurred at sea; on average, 31.5 ± 12.4% of the first-year mortality was due to neonate death on the rookery. Juvenile survivorship rates were lowest in El Niño years (1978, 1983, and 1986). As colony size increased fivefold over the study period, survivorship to age 1 did not change significantly, but survivorship to age 4 decreased significantly. No significant relationship was found between weanling mass and survival to 1 and 2 years of age. Survivorship to 1 year of age was positively correlated with standard length, but this relationship did not hold for survivorship to age 2.
The juvenile survivorship rate of seals from the Año Nuevo colony is too low to support the observed growth rate of the colony and of the population as a whole. Other California rookeries, such as San Miguel Island, must have significantly higher juvenile survivorship rates to account for the recent population increase. The causes of high juvenile mortality at sea are unknown; they do not appear to be related to condition at weaning, as reflected by weaning weight.
Survivorship and fertility schedules shape life history tactics (Stearns 1976, 1980) and provide vital demographic data for estimating population growth (Wilson and Bossert 1971). This chapter addresses juvenile survivorship in northern elephant seals, the percentage of individuals born that survive to each of the first four years of life. Our aim is to describe age-specific survival rates and age-specific mortality rates of seals born at Año Nuevo, California, over the last two decades when colony size was increasing and to examine the role of year, cohort, and condition at weaning—as reflected by weight, length, or an index of the two measures—on survival. Because juvenile survivorship is an important determinant of the growth or decline of a population, data presented here may elucidate the rapid growth of the northern elephant seal population over the last few decades as well as provide an instructive comparison with southern elephant seals, whose numbers are declining at several rookeries (Hindell, Slip, and Burton, this volume).
This chapter summarizes and augments data on juvenile survivorship of Año Nuevo-born seals presented in J. Reiter, N. L. Stinson, and B. J. Le Boeuf (1978), J. Reiter (1984), B. J. Le Boeuf and J. Reiter (1988), and B. J. Le Boeuf and J. Reiter (1991).
Background
Año Nuevo is a peripheral colony in the northern elephant seal range. Since breeding began here in 1961 (Radford, Orr, and Hubbs 1965), it has received immigrants from larger southern rookeries in southern California, San Miguel and San Nicolas islands. Throughout this period, the entire population has grown steadily (Stewart et al., this volume). Births at the Año Nuevo colony have increased at the rate of 14% per year, and annual pup production is now on the order of 2,000 pups. The growth, however, is due mainly to immigration from southern rookeries, for internal recruitment is too low for the colony to sustain itself (Le Boeuf and Reiter 1988).
Most females give birth for the first time at age 4 (range 3–6 years of age) and then give birth annually until death. Single pups are produced, nursed 25 to 28 days, and weaned abruptly when the mother returns to sea. The weaned pup fasts for 2½ months on the rookery while learning to swim and dive before going off on its first foraging trip (Reiter, Stinson, and Le Boeuf 1978).
Juveniles make two foraging trips per year, each lasting about five months (see fig. 13.1 in chap. 13). As a result, they appear on the rookery twice a year, in the spring and in the fall, each haul-out lasting about one month. At this time, they are identified and survival is estimated. The pattern changes when females begin giving birth. Consequently, sex differences
in survival begin to appear in year 3, partially a result of a sex difference in time spent at sea.
Methods
Pups born in the years 1971 to 1988 at Año Nuevo, California, were tagged shortly after weaning with one or two cattle ear tags in the interdigital webbing of the hind flippers (Le Boeuf and Peterson 1969). The number of seals tagged per year varied from 100 to 900. Survivors were identified when their tags were read at approximately weekly intervals on the island and the mainland resting and molting sites at Año Nuevo. Seals dispersing to other rookeries were identified and reported to us by H. Huber and W. Sydeman for Southeast Farallon Island, S. Allen for Point Reyes Headlands, and B. Stewart and R. DeLong for San Miguel and San Nicolas islands. Seals that stranded along the central California coast were reported to us by researchers at the California Marine Mammal Center.
From resights of tagged seals, we calculated life tables to age 4. The criterion for survival to age 1 was resighting the seal after the first trip to sea, when it was 9 to 10 months old in the fall or 15 to 17 months old in the spring. Survival to age 2, 3, and 4 was recorded similarly. In our experience, only slightly more juveniles are observed in the spring than in the preceding fall haul-out.
The survivorship data for each cohort and age class were adjusted to account for unrecognizable survivors that lost their tag identification. The proportion of animals that lost their tags varied from year to year because cohorts varied with respect to the proportion of single- and double-tagged individuals. Nearly all seals in the cohorts during the interval 1985–1988 were double tagged. For single-tagged seals, we assumed a tag loss rate of 11% per annum for the first two years of life and 6% per annum thereafter. The tag loss rate of double-tagged seals was assumed to be the rate of single tag loss squared, or 1.21% per annum for the first two years and 0.36% per annum thereafter. Tag loss estimates were based on the loss rate of single tags determined from double-tagged individuals (Reiter 1984)
The influence of weight, length, and a condition index on the probability of first-year survival was investigated. During 1978 and the years 1984–1988, 734 weaned pups were weighed and measured within a month of weaning. Weaning mass was estimated by back calculation based on known rates of mass lost per day (see equation in Appendix 10.1 of Deutsch et al., this volume). All pups that weighed less than 50 kg were excluded from the analysis. These were orphaned pups that did not suckle normally; most of them died on the rookery or stranded nearby shortly after going to sea. Standard length was measured in a straight line from tip of nose to tip of
| |||||||||||||||||||||||||||||||||||
tail above the dorsal surface. A condition index, ostensibly reflecting a pup's stored energy reserves, was calculated as mass divided by length.
Results
Age-specific Survival
Of the pups born during the years 1971–1988, the mean percentage that survived to age 1 was 36.8 ± 8.5; to age 2, 26.3 ± 6.3; to age 3, 19.4 ± 5.1; and to age 4, 16.3 ± 5.2. These data are presented as partial life tables in table 7.1 and figure 7.1. Age-specific survival varied widely over the years, with the following range of values being observed: 19.9–48.7% to age 1; 11.1–37.4% to age 2; 7.1–28.9% to age 3; and 5.0–26.5% to age 4. Survival rates were highest for the year 1971 and lowest for the year 1983 (fig. 7.2).
Age-specific Mortality
Age-specific mortality for the entire sample was highest during the first year of life, 63.2%, and then dropped steadily until reaching a low of 16% between 3 and 4 years of age (table 7.1, fig. 7.1).
Mortality on the Rookery and at Sea during the First Year
Over the study period, a mean of 31.5 ± 12.4% of the first-year mortality was due to neonate death on the rookery; the majority of the first-year mortality occurred at sea. The proportion of first-year mortality occurring on the rookery reached a high of 61% of pups born in 1983 due to the rookery being inundated by storm-whipped high surf at high tide during the peak
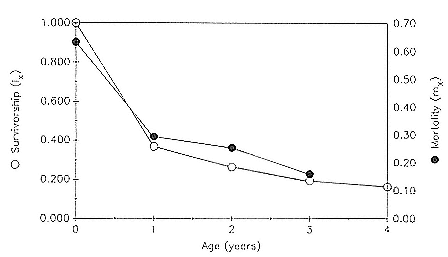
Fig. 7.1
Survivorship (lx ) and mortality (mx ) curves for the northern elephant seal at
Año Nuevo, California, during the years 1971–1988. Based on data in table 7.1.
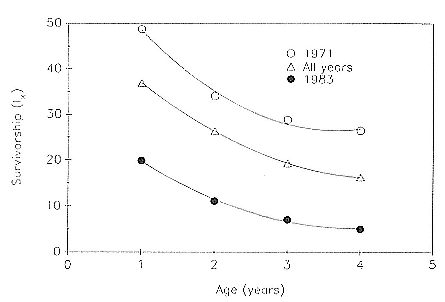
Fig. 7.2
Survivorship (lx ) curves for northern elephant seals from Año Nuevo, California,
during the best (1971) and worst (1983) years in the study period, 1971–1988,
and for all years combined.
pupping period (see also Le Boeuf and Condit 1983; Le Boeuf and Reiter 1991).
Survival Rates of Weanlings
For all years combined, the mean survival rate of weanlings over the periods at sea was 46.0 ± 7.7% (range = 35.0%–61.1%) to age 1; 32.8 ± 5.9% (range = 21.6–44.5%) to age 2; 24.2 ± 5.5% (range = 13.8–33.8%) to 3; and 20.3 ± 5.8% (range = 9.8–30.9%) to age 4.
The Effect of Year and Associated Conditions
The role of a specific year and associated conditions, such as weather and prey availability, is reflected by the annual survival rates of 1-, 2-, 3- and 4-year-olds in that year. For example, in 1981, survival to age 1 was 42.5 ± 4%; the survival rate of the 1980 cohort from age 1 to 2 during 1981 was 91.9%; the 1979 cohort survival from age 2 to 3 in 1981 was 96.0%; and the 1978 cohort survival from age 3 to 4 in 1981 was 97.5%, giving 1981 a mean score of 82.0%.
Calculated in this way, the mean score of all cohorts was 79.1 ± 3.6%. There were three years with mean scores one standard deviation or more above the mean, an indication that they were exceptionally good years: 1974 (84.6%), 1985 (83.3%), and 1980 (82.6%). There were three poor years: 1983 (72.7%), 1986 (74.0%), and 1978 (76.0%). All three poor years are categorized as El Niño years by oceanographers.
Cohort Variation
As the size of the Año Nuevo colony increased more than fivefold from 1971 to 1988 (an increase similar to that of the entire population; see Stewart et al., this volume), one might expect lower survivorship values in the later years due to increased competition for resources either on the rookery or on the foraging grounds. Pup mortality on the island prior to weaning increased from a low of 14.5% of pups born in 1971 to a high of 70% of pups born in 1983; preweaning pup mortality is density dependent, and there is a significant interaction with weather (Le Boeuf and Briggs 1977; Le Boeuf and Reiter 1991). There was no tendency for survivorship to age 1 to decrease with time and increasing density (fig. 7.3a); however, survivorship to age 4 decreased with time (the regression of y on x = 90.1 – 0.94x; r = 0.81).
An indication of the relative long-term strength of a cohort is the percentage decrease in the juvenile survival rate from year 1 to year 4. The percent decrease in survivorship from age 1 to age 4 increased over the study period (fig. 7.3b); that is, the mortality rate over the juvenile years increased with time.
The mean percentage decrease in survivorship from age 1 to 4 (fig. 7.3b)
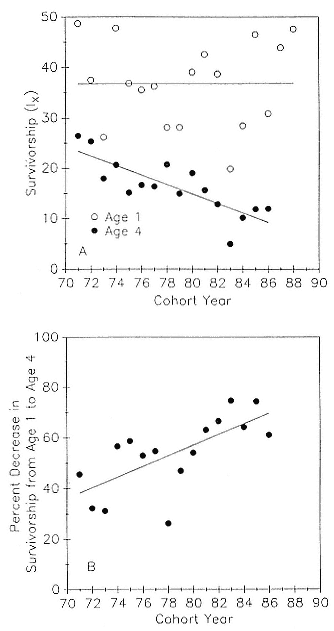
Fig. 7.3
(A) Survivorship to age 1 (open circles) and to age 4 (closed
circles) as a function of cohort year. The regression equation
for the latter is y = 90.1 – 0.94x; r = .81. (B) The percent
decrease in survivorship from age 1 to age 4 as a function
of cohort year. The regression equation is
y = –111 + 2.1x; r = .69.
for all 18 cohorts was –55.2 ± 14.1%. The three strongest cohorts, those with the lowest percentage decline over the three-year period, were 1978 (–26.2 ± 3%), 1973 (–31.3%), and 1972 (–32.3%). The three weakest cohorts were 1983 (–74.4%), 1985 (–74.4%), and 1988 (–66.9%).
The Effect of Weaning Mass, Size, and Condition on Age-specific Survival
Although the weight of weanlings varied greatly, with animals in the highest weight category being almost twice as large as those in the lowest weight category (fig. 7.4a), there was no significant relationship between mass at weaning and survival to 1 year of age (chi-square = 8.17, df = 8, p > .05) or to 2 years of age (chi-square = 6.83, df = 8, p > .05) (fig. 7.4b). Seals with the most common weights at weaning, in the range of 120 to 150 kg, had the lowest survivorship to year 1, 34.8 to 41.7%). However, these rates did not differ significantly from that of other weight categories. The extreme low and high weight categories incurred the greatest decline in survivorship from year 1 to year 2, but these differences, too, were not significantly different from the declines in other weight categories (chi-square = 8.53, df = 8, p > .05).
Survivorship to 1 year of age varied significantly as a function of standard length at weaning (chi-square = 12.9, df = 8, p < .05)—weanlings with the smallest standard lengths had the lowest survivorship—but this effect did not hold for survival to age 2 (fig. 7.5). Survivorship did not vary significantly with condition index.
Reasoning that high weight or great size might be advantageous in a poor year, we examined separately the year 1986, the only year in the weighed weanling sample that physical oceanographers categorize as an El Niño year. During El Niño years, foraging may be more difficult because of lower prey availability (Arntz, Pearcy, and Trillmich 1991). Using the same weight and length classes shown in figures 7.4 and 7.5, survivorship of the 1986 cohort did not vary significantly with any measure of condition.
Discussion
Northern elephant seals exhibit the most common survivorship curve in nature, Type III, which is characterized by a steep decline in survivorship at an early age (Wilson and Bossert 1971). Those that survive the juvenile period have a good chance of reaching maturity. The majority of young northern elephant seals that were born at Año Nuevo during this study (nearly two-thirds of them, on average) did not survive to 1 year of age, and only 20%, on average, lived to age 4. Most of the juvenile mortality to age 1 occurs at sea, and all mortality to age 2, 3, and 4 occurs at sea. White sharks, Carcharodon carcharias , and killer whales, Orcinus orca , are known predators on northern elephant seals (Ainley et al. 1981; Le Boeuf, Riedman,
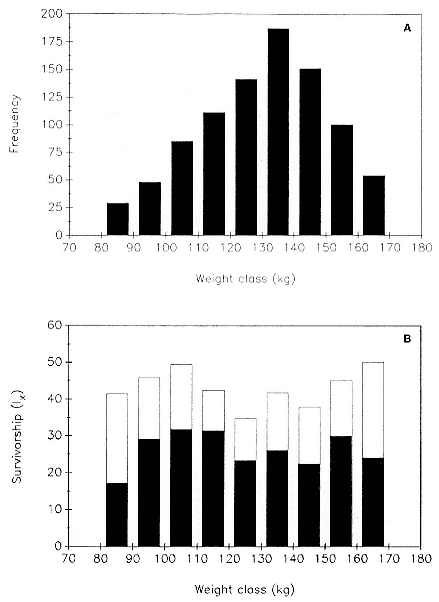
Fig. 7.4
(A) Frequency distribution of 734 northern elephant seal pups by weight class
at weaning. (B) Survivorship of northern elephant seal juveniles to age 1
(open bars) and to age 2 (closed bars) as a function of their weight class at
weaning. The sample is taken from Año Nuevo, California,
during the years 1978 and 1984–1988.
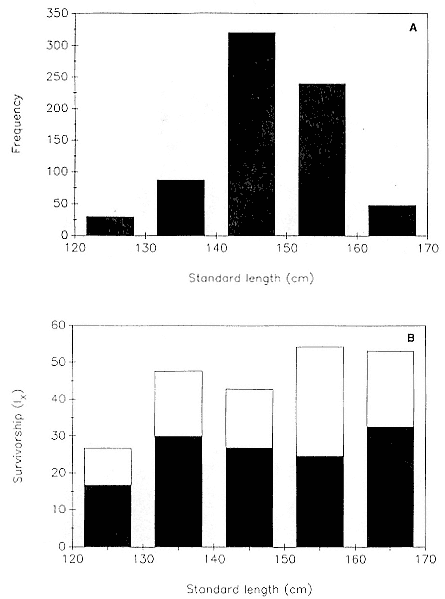
Fig. 7.5
(A) Frequency distribution of 734 northern elephant seal pups by standard length
class at weaning. (B) Survivorship of juveniles to age 1 (open bars) and to age 2
(closed bars) as a function of standard length at weaning.
and Keyes 1982; M. Pierson, pers. comm.), but the degree to which they account for the observed mortality rates is not clear.
In any case, the high juvenile mortality rate at Año Nuevo does not appear to be due to condition at weaning, insofar as condition is reflected by mass at weaning. Weaning weight (above 80 kg) was not correlated with survival to age 1 or to age 2. The fattest were not the fittest (see Sinervo et al. 1992). This is surprising given the large body of information on the importance of parental investment in enhancing individual reproductive success through the production of progeny (e.g., Clutton-Brock 1991). It remains to be determined whether mass at weaning is correlated with reproductive success.
Like other northern and southern elephant seal colonies (Huber, Beckham, and Nisbet 1991; Hindell 1990), cohort variation in juvenile survivorship at Año Nuevo was great, especially to age 1. Much of this variability was due to the effect of storms that occurred during the peak pupping period and caused high pup mortality on the rookery. A decrease in survivorship from age 1 to age 4 was evident over the course of the study period. This may have been due to increasing competition as the population grew or to interactions with fisheries, for example, high seas drift net fisheries in foraging areas. The cause of this decline may become clear as we begin to accumulate knowledge of the migratory paths and foraging areas of juveniles (Le Boeuf, this volume).
The juvenile mortality rate of Año Nuevo-born elephant seals is high relative to other well-studied large mammals such as Dall mountain sheep, Ovis dalli (Deevey 1947), and red deer, Cervus elephus (Clutton-Brock, Albon, and Guinness 1988), but strikingly similar to the low rates of male, relative to female, African lions, Panther leo , from the Serengeti population (Packer et al. 1988) and male vervet monkeys, Cercopithecus aethiops (Cheney et al. 1988).
Juvenile survivorship of northern elephant seals from the Año Nuevo colony is significantly lower than that of southern elephant seals from South Georgia, a large colony that was stable in numbers during the period 1951–1985 (Laws, this volume). T. S. McCann (1985) revised the life tables of R. M. Laws (1960) and estimated survivorship to age 1 as 60%, to age 2 as 51%, to age 3 as 43.5%, and to age 4 as 37.2% (we combined the sexes in his life tables for comparability). In comparison with this Southern Hemisphere rookery, juvenile survivorship at Año Nuevo was 42% lower to age 1 and 52% lower to age 4. Estimates of juvenile survivorship at Marion Island are similar to those at South Georgia, despite declines in number at the rate of 4.5% per year during the period 1974–1989 (Bester and Wilkinson, this volume).
Juvenile survivorship rates from Año Nuevo more closely resemble those of the declining southern elephant seal colony at Macquarie Island, studied
by R. Carrick and S. E. Ingham (1962) and M. A. Hindell (1990). Hindell (1991, this volume) estimated first-year survival at Macquarie Island during the 1950s as 44% (both sexes combined); however, from 1960 to 1965, first-year survival declined dramatically to 2%. Life table estimates of survival to each of the first four years of life (1x ), based on the entire study period, are 0.350, 0.298, 0.232, and 0.178, respectively. These values are similar to those reported in table 7.1 for Año Nuevo: 0.368, 0.263, 0.194, and 0.163.
These results are paradoxical, or at least do not appear to have a unitary explanation. Despite equally high juvenile survivorship rates, the Año Nuevo colony is increasing in number and the Macquarie Island colony is declining in number. Why is Marion Island declining in number despite a high juvenile survivorship rate? This state of affairs is not easily explained given current information. However, the following information is important for sorting out these incongruencies.
1. Año Nuevo may not be the ideal representative of an expanding colony for comparison with stable or declining colonies. The increase in the Año Nuevo colony, and other colonies like the Farallons at the northern boundary of the species' breeding range, is due mainly to dispersion and immigration from large rookeries in southern California, especially San Miguel Island; internal recruitment, alone, would lead to a decline in colony numbers (Le Boeuf and Reiter 1988; Huber, Beckham, and Nisbet 1991). San Miguel Island accounts for most of the growth of the entire northern elephant seal population (Stewart et al., this volume) and hence may best represent an expanding population. Presumably, juvenile survival rates at San Miguel Island are higher than those at Año Nuevo and perhaps even higher than those at South Georgia. Unfortunately, there are no data on juvenile survivorship for this rookery.
2. Considerable mixing occurs between colonies in the Northern Hemisphere. Consequently, the factors that make for growth of northern elephant seal colonies are more difficult to assess than those of southern elephant seal colonies, where immigration is rare or nonexistent (Burton 1985; Bester 1989; Gales, Adams, and Burton 1989; Hindell 1990) and growth depends ultimately on internal recruitment. For example, during the period 1969–1976, there was considerable dispersion of northern elephant seals among the seven extant rookeries (Bonnell et al. 1979). Movement was primarily in the northward direction and most prevalent during the first year of life. The majority of the movements represented permanent immigration. Southern California rookeries received immigrants from Mexican rookeries and sent out immigrants to northern California rookeries. There was
bidirectional exchange between rookeries separated by short distances, such as San Miguel and San Nicolas islands in southern California and Año Nuevo and the Farallons in central California.
3. Differences in adult female mortality might partially explain the different population trajectories of the expanding northern elephant seal population as compared to stable or declining colonies in the Southern Hemisphere. Adult female mortality is apparently lower at northern elephant seal rookeries such as Año Nuevo and the Farallons (Le Boeuf and Reiter 1988; Huber et al. 1991) than at the southern elephant seal rookery at Marion Island, where it is concluded that the colony decline is due mainly to high adult female mortality (Bester and Wilkinson, this volume). Natality of adult females does not appear to account for differences in growth of colonies since these rates are uniformly high at colonies in both hemispheres; that is, natality exceeds 85% of adult females (McCann 1985; Le Boeuf and Reiter 1988; Huber et al. 1991; Bester and Wilkinson, this volume). Longevity of females seems to be substantially greater at Año Nuevo than at Macquarie, but the data are from different eras and are not a fair comparison.
4. Differences in methodology may cause substantial variation in estimates of juvenile survivorship rates. For example, survivorship rates at Macquarie Island were determined by monitoring animals branded at weaning, while those at Año Nuevo and Marion Island were based on recovery of animals marked with cattle ear tags. Branding yields a permanent mark; tags are impermanent, and some of them are lost. When tags are used, the estimate of juvenile survivorship is affected by the estimate of tag loss. Additionally, tags are harder to see and read than brand marks. Consequently, tag loss is usually greater than estimated, leading to an underestimate of survivorship. That is, the survivorship rates based on tag resight data we have presented for the Año Nuevo colony are probably minimum estimates.
The pup mortality rate on the rookery before weaning, when the animals are marked, is another variable that affects estimates of juvenile survivorship. Deaths on the rookery are a component of the initial sample size. Neonate deaths on small rookeries like Año Nuevo can be counted directly or calculated with reasonable confidence from censuses of suckling and weaned pups. The mean preweaning pup mortality rate at Año Nuevo during the present study was 24.4 ± 10.7% of pups born. On large rookeries such as Macquarie Island, the preweaning pup mortality rate is assumed to be the rate observed in selected harems amenable to censusing. This rate was assumed to be 4.5% of pups born for South Georgia (McCann 1985)
and 5% of pups born for Macquarie Island (Hindell and Burton 1987). To what extent these different methodologies explain the wide disparity in preweaning pup mortality rates is not clear. A similar statement could be made about search effort, which, necessarily, varies with the size, terrain, and location of rookeries.
In summary, one can obtain reasonably accurate estimates of juvenile survivorship from small, expanding northern elephant seal colonies, such as Año Nuevo, but the extent to which they elucidate the role of juvenile survivorship in declining colonies is unclear. Indeed, Año Nuevo would be declining at a similar rate as Macquarie Island were it not for the influx of animals from San Miguel Island. It may be more important to document juvenile survivorship at San Miguel Island because its growth rate drives the growth of the population. But the task is made difficult by the sheer size of the colony and the need to estimate immigration and emigration rates.
Indeed, the most appropriate comparison, if not the easiest, is to compare the entire northern elephant seal population with that of either of the three main southern elephant seal populations defined by Laws (1960) as the South Georgia stock, the Kerguelen stock, and the Macquarie Island stock (see fig. 3.1, chap. 3). Like the northern elephant seal population, each southern stock is geographically isolated; animal movements between stocks are rare, and gene flow is limited (Gales, Adams, and Burton 1989). Animal movements within each population have an important effect on juvenile survivorship and female reproductive success and, ultimately, on population regulation.
References
Ainley, D. G., C. S. Strong, H. P. Huber, T. J. Lewis, and S. H. Morrell. 1981. Predation by sharks on pinnipeds at the Farallon Islands. Fishery Bulletin, U.S. 78: 941–945.
Arntz, W., W. G. Pearcy, and F. Trillmich. 1991. Biological consequences of the 1982–83 El Niño in the Eastern Pacific. In Pinnipeds and El Niño: Responses to Environmental Stress , ed. F. Trillmich and K. Ono, 22–24. Berlin: Springer Verlag.
Bester, M. N. 1989. Movements of southern elephant seals and subantarctic fur seals in relation to Marion Island. Marine Mammal Science 5: 257–265.
Bonnell, M. L., B. J. Le Boeuf, M. O. Pierson, D. H. Dettman, and G. D. Farrens. 1979. Summary Report, 1975–1978. Marine Mammal and Seabird Surveys of the Southern California Bight Area . III. Pinnipeds . Bureau of Land Management, Department of the Interior, Contract AA550-CT7-36.
Burton, H. R. 1985. Tagging studies of male southern elephant seals (Mirounga leonina L.) in the Vestfold Hills area, Antarctica, and some aspects of their behavior. In Sea Mammals of South Latitudes: Proceedings of a Symposium of the 52d ANZAAS Congress in Sydney—May 1982 , ed. L. K. Ling and M. M. Bryden, 19–30. Northfield: South Australian Museum.
Carrick, R., and S. E. Ingham. 1962. Studies on the southern elephant seal, Mirounga leonina (L.). V. Population dynamics and utilization. CSIRO Wildlife Research 7: 198–206.
Cheney, D. L., R. M. Seyfarth, S. J. Andelman, and P. C. Lee. 1988. Reproductive success in vervet monkeys. In Reproductive Success , ed. T. H. Clutton-Brock, 384–402. Chicago: University of Chicago Press.
Clutton-Brock, T. H. 1991. The Evolution of Parental Care . Princeton: Princeton University Press.
Clutton-Brock, T. H., S. D. Albon, and F. E. Guinness. 1988. Reproductive success in male and female red deer. In Reproductive Success , ed. T. H. Clutton-Brock, 325–343. Chicago: University of Chicago Press.
Deevey, E. S., Jr. 1947. Life tables for natural populations of animals. Quarterly Review of Biology 22: 263–314.
Gales, N. J., M. Adams, and H. R. Burton. 1989. Genetic relatedness of two populations of the southern elephant seal, M. leonina. Marine Mammal Science 5: 57–67.
Hindell, M. A. 1990. Population dynamics and diving-behaviour of a declining population of southern elephant seals. Ph.D. dissertation, University of Queensland, Australia.
———. 1991. Some life history parameters of a declining population of southern elephant seals, Mirounga leonina. Journal of Animal Ecology 60: 119–134.
Hindell, M. A., and H. R. Burton. 1987. Seasonal haul-out patterns of the southern elephant seal (Mirounga leonina ) at Macquarie Island. Journal of Zoology, London 213: 365–380.
Huber, H. R., C. Beckham, and J. Nisbet. 1991. Effects of the 1982–83 El Niño on northern elephant seals on the South Farallon Islands, California. In Pinnipeds and El Niño: Responses to Environmental Stress , ed. F. Trillmich and K.A. Ono, 219–233. Berlin: Spring Verlag.
Huber, H. R., A. C. Rovetta, L. A. Fry, and S. Johnston. 1991. Age-specific natality of northern elephant seals at the South Farallon Islands, California. Journal of Mammalogy 72: 525–534.
Laws, R. M. 1960. The southern elephant seal (Mirounga leonina Linn.) at South Georgia. Norsk Hvalfangst-Tidende 49: 466–476, 520–542.
Le Boeuf, B. J., and K. T. Briggs. 1977. The cost of living in a seal harem. Mammalia 41: 167–195.
Le Boeuf, B. J., and R. S. Condit. 1983. The high cost of living on the beach. Pacific Discovery 36: 12–14.
Le Boeuf, B. J., and R. S. Peterson. 1969. Social status and mating activity in elephant seals. Science 163: 91–93.
Le Boeuf, B. J., and J. Reiter. 1988. Lifetime reproductive success in northern elephant seals. In Reproductive Success , ed. T. H. Clutton-Brock, 344–362. Chicago: University of Chicago Press.
———. 1991. Biological effects associated with El Niño Southern Oscillation, 1982–83, on northern elephant seals breeding at Año Nuevo, California. In Pinnipeds and El Niño: Responses to Environmental Stress , ed. F. Trillmich and K. Ono, 206–218. Berlin: Springer Verlag.
Le Boeuf, B. J., M. Riedman, and R. S. Keyes. 1982. White shark predation on pin-
nipeds in California coastal waters. Fishery Bulletin 80: 891–895.
McCann, T. S. 1985. Size, status and demography of southern elephant seal (Mirounga leonina ) populations. In Sea Mammals of South Latitudes: Proceedings of a Symposium of the 52d ANZAAS Congress in Sydney—May 1982 , ed. J. K. Ling and M. M. Bryden, 1–17. Northfield: South Australian Museum.
Packer, C., L. Herbst, A. E. Pusey, J. D. Bygott, J. P. Hanby, S. J. Cairns, and M. B. Mulder. 1988. Reproductive success of lions. In Reproductive Success , ed. T. H. Clutton-Brock, 363–383. Chicago: University of Chicago Press.
Radford, K. W., R. T. Orr, and C. L. Hubbs. 1965. Reestablishment of the northern elephant seal, Mirounga angustirostris , off central California. Proceedings of the California Academy of Sciences 31: 601–612.
Reiter, J. 1984. Studies of female competition and reproductive success in the northern elephant seal. Ph.D. dissertation, University of California, Santa Cruz.
Reiter, J., N. L. Stinson, and B. J. Le Boeuf. 1978. Northern elephant seal development: The transition from weaning to nutritional independence. Behavioral Ecology and Sociobiology 3: 337–367.
Sinervo, B., P. Doughty, R. B. Huey, and K. Zamudio. 1992. Allometric engineering: A causal analysis of natural selection on offspring size. Science 258: 1927–1930.
Stearns, S. C. 1976. Life history tactics: A review of ideas. Quarterly Review of Biology 51: 3–47.
———. 1980. A new view of life-history evolution. Oikos 35: 266–281.
Wilson, E. O., and W. H. Bossert. 1971. A Primer of Population Biology . Stamford, Conn.: Sinauer Associates.