7.1—
Introduction
Microtubules were first described in spermatozoids of the moss, Sphagnum, by Irene Manton in 1957. Since the introduction, six years later, of glutaraldehyde as a fixative for electron microscopy, their presence has been revealed in a very wide variety of plant and animal cells. They have not been observed in prokaryotes.
These organelles are associated with several processes in the repertoire of movements exhibited by the eukaryotic cell, including; movements of individual cells by flagellar motion or axopod extension; movement of components within cells, as in mitosis, axoplasmic flow or transport of certain pigment granules; and morphogenetic movements involving the generation and maintenance of cell shape. In the case of plant cells, in addition to chromosome movements during cell division, the presence of microtubules has been correlated with definition of the plane of cell cleavage, formation of the cell plate and determination of cell wall architecture.
In certain cell types, microtubules are seen only during mitosis and meiosis, suggesting that they were involved originally in these processes alone, and only later did they come to be used for extranuclear functions in advanced eukaryotic organisms. Indeed, it has been suggested (Margulis, 1970) that acquisition of the microtubule was a major factor in permitting the evolution of the eukaryote cell.
7.1.1—
Description
Under the electron microscope, microtubules appear as long, unbranched cylindrical structures with a diameter of 22–25 nm (Fig. 7.1 and Fig. 7.4). In transection, they show an electron-lucent core, ca. 12 nm in diameter, and a wall of, usually, 13 electron-dense subunits. The subunits are 4–5 nm wide and are organized along the long axis of the microtubule into protofilaments. There is an axial displacement of subunits between adjacent protofilaments, resulting in a visible pitch relative to the long axis of the tubule (Fig 7.2).
Microtubules are usually separated from each other or from adjacent cellular components by a clear space of 10–40 nm. Also, in longitudinal view, an electron-lucent zone is often observed along the sides of the microtubule. These observations suggest that each tubule may be surrounded by a specialized region or layer of material. In certain systems, microtubules show 'arms' projecting from the walls of the tubules at regular intervals along their length.
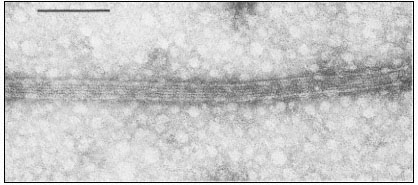
Figure 7.1
Negatively stained microtubules prepared from brain, showing axially aligned
protofilaments and their substructural periodicity. Scale marker 0.1 m m.
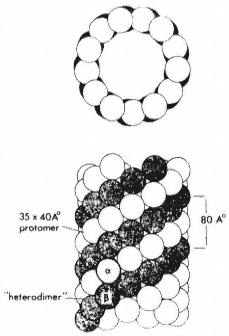
Figure 7.2
Model microtubule, showing in transection the 13 globular
subunits comprising the wall, and in longitudinal view, the
axial displacement of subunits in adjacent protofilaments. From
Bryan (1974) Fedn. Proc. Fedn. Am. Socs. exp. Biol. 33, 152–57,
reproduced by permission of the author and Fedn. Am. Socs. exp. Biol.
Such lateral projections act, in some instances, as cross-bridges between adjacent microtubules. Alternatively, projections can extend from microtubules to adjacent membranes such as the plasmalemma, the nuclear envelope, the endoplasmic reticulum or associated vesicles.
7.1.2—
Background
Eukaryote mitotic spindles are a heterogeneous group of intracellular structures characterized by the presence of anisotropically arranged microtubules. The number of microtubules within the spindle of a given species appears to reflect the mass of the chromosomes, and can vary from a few to several thousand. The highly oriented microtubules are responsible for the weak form-birefringence of the spindle under polarized light and there are natural fluctuations in spindle birefringence during the different phases of mitosis.
Mitotic arrest can be achieved with a variety of physical and chemical agents. Spindle birefringence is also affected by such agents. For example, birefringence in dividing Lilium pollen mother cells is abolished within seconds of exposure to low temperature. Upon return to normal temperatures, spindle birefringence reappears in a few minutes, after which mitosis proceeds normally. Exposure to high hydrostatic pressure causes a similar effect. Chemical agents with antimitotic activity, such as the alkaloids colchicine, podophyllotoxin or vinblastine, also abolish spindle birefringence. The important feature of all these effects is that they are reversible upon removal of the inhibitory agent.
Based on: (a) natural fluctuations in spindle birefringence, (b) reversible effects of inhibitors on both birefringence and mitosis and (c) the findings of many earlier studies that the major components of the spindle are synthesized prior to prophase, Inoué and Sato in 1967 proposed a model in which the spindle is envisaged as a labile structure in dynamic equilibrium with a pool of subunits. Mitosis is thus a process which reflects the sequential assembly and disassembly of different structures to perform different functions. Inhibitory agents may act by disrupting the dynamic equilibrium. This model had a profound effect in engendering the concept of certain microtubules being labile structures capable of being polymerized or depolymerized under cellular control (Tilney, 1971).
However, several lines of evidence suggest that there are different classes of microtubules. Morphological variations in arrangement and in associated components have already been briefly described. Perhaps more importantly, microtubules of cilia or flagella are not depolymerized by treatments which reversibly destroy the birefringence of the spindle. Similarly, certain cytoplasmic microtubules of higher plant cells appear to be resistant to anti-mitotic chemicals. The generalization has been made that microtubules be classified as stable, i.e. those in, for example, cilia and flagella, or labile, i.e. cytoplasmic microtubules in many types of animal cells (Margulis, 1973).