21—
RIPARIAN SYSTEMS AND THE ECOLOGY OF NON-AVIAN WILDLIFE POPULATIONS

Ringtail Distribution and Abundance in the Central Valley of California[1]
Linda Belluomini and Gene R. Trapp[2]
Abstract.—The presence of ringtails (Bassariscusastutusraptor ) in riparian systems of the northern Central Valley is documented in this study, constituting a range extension. Ringtail densities 2.5 to 5 times greater than reported in the literature for other habitats were recorded, viz . 10.5 to 20.5 per 100 ha. (26.7 to 52.8 per mi2 ). Habitat composition was examined and related to the densities obtained. A direct relationship is suggested between ringtail density and community productivity and structural complexity.
Introduction
Ringtails (Bassariscusastutus ) are distributed throughout 11 southwestern states and portions of Mexico (Long and House 1962; Hall 1981). Throughout their range they are typically associated with boulder-strewn chaparral, chaparral interspersed with evergreen woodland, oak woodland, and scrub vegetation of various types (Seton 1929; Grinnell etal . 1937; Taylor 1954; Davis 1960; Hall 1981). Within California, ringtails inhabit the "Upper Sonoran Life Zone" on Pacific drainage slopes from the Oregon border to Mexico and the mountain ranges east of the Southern Sierra Nevada (Grinnell etal . 1937). Although acknowledging the presence of ringtails (the race B . a . raptor ) in streamside vegetation to about the 160-m. (500ft.) elevation, Grinnell etal . (ibid .) apparently found no evidence to suggest that ringtails occurred in this vegetation-type on the floor of the Central Valley. However, they did report ringtail observations for the Sacramento River in southern Tehama County, in the northern end of the Valley. Their belief that ringtails were restricted to the surrounding foothills and mountains is apparent in their distribution map.
The first published report of ringtails occurring on the Valley floor was made by Naylor and Wilson (1956). They observed ringtails in Wood Duck (Aixsponsa ) nest boxes in Butte Sink, along Butte Creek, 4.8 km. (3 mi.) north-east of Colusa, Colusa County, on the floor of the Sacramento Valley. Notwithstanding the recognized occurrence of ringtails in Butte Sink (Hall and Kelson 1959; Schempf and White 1977; Hall 1981), reviews of ringtail distribution (op. cit.; Ingles 1965) subsequent to Grinnell etal . (1937) did not modify the range of ringtails in California relative to the Central Valley.
In December 1971, Dr. Dallas Sutton[3] informed Trapp about the ease of collecting ringtails along the Sacramento River southwest of Chico for his mammal collection at California State University at Chico. Subsequently, two recent mammal inventories suggested that ringtails may occur in riparian vegetation associated with the network of drainages in the Central Valley. Stone (1976) detected an abundance of ringtails at certain sites along the Sacramento River in Tehama County at localities approximating those reported to have ringtails by Grinnell etal . (1937). Also, while conducting a mammal census of the Bobelaine Audubon Sanctuary on the Feather River at its confluence with the Bear River in Sutter County, during the spring of 1978, members of the Ecological Research Society of California State University, Sacramento, livetrapped eight ringtails. These data also suggested ringtail densities in riparian vegetation greater than those reported in the literature for other types of systems (ibid .; Taylor 1954; Trapp 1978).
The present study had three objectives: 1) to refine earlier distribution studies of the ringtail in California with respect to the Central Valley; 2) to verify preliminary observa-
[1] Paper presented at the California Riparian Systems Conference. [University of California, Davis, September 17–19, 1981].
[2] Linda Belluomini is Resource Specialist, Natural Resources, Marine Corps Base, Camp Pendleton, Calif. Gene R. Trapp is Associate Professor of Biological Sciences, California State University, Sacramento.
[3] Dr. Dallas Sutton, Emeritus Professor, California State University, Chico. Personal conversation.
tions which indicated desities much higher in riparian vegetation of the Central Valley than reported in the literature for other vegetation-types occupied by ringtails; and 3) to examine the composition of the riparian plant community associated with sites occupied by ringtails.
Methods and Materials
Ringtail distribution in the Central Valley was evaluated using five methods: 1) a literature review was conducted; 2) a furbearer observation questionnaire was circulated statewide; 3) locality data were gleaned from museum specimens; 4) knowledgeable persons were contacted; and 5) ringtails were captured by livetrapping.
Furbearer observation reports requesting specific information on ringtail sightings were distributed to appropriate Federal, State, and local agencies (e.g., wildlife refuges, sanctuaries, and preserves; California Department of Fish and Game biologists and wardens), as well as licensed trappers throughout California. Letters requesting pertinent locality information were sent to selected college and university museums which might have on deposit ringtail skins and/or skulls collected in the Valley. Personal contact was initiated with persons known to be familiar with wildlife of the Central Valley. Livetrapping was conducted during the summer and fall of 1978 and 1979 at nine sites in the Sacramento Valley to determine if ringtails were present.
Ringtails were easily captured using singledoored 9x9x26-in. and double-doored 6x6x24-in. galvanized, wire mesh livetraps.[4] Several baits (strawberry jam, raisins, muskrat meat) and ringtail lure were used to attract ringtails into the traps. Strawberry jam was the most effective attractant.
Data used for computation of density were collected by livetrapping, tagging, and recapture at five of the nine sites mentioned above. Livetraps were placed in a nonrandom manner (i.e., adjacent to ringtail scats; on, or alongside logs, etc.); throughout a portion of a particular study site. When recaptures indicated that all individuals within the immediate vicinity had been caught, the traps were moved variable distances away from that location until unmarked individuals were captured. This direct count process was repeated until presumably all ringtails within a study site were trapped. Aerial photographs were planimetered to determine the area of each study site, excluding habitats where ringtails were not captured or areas thought to be non-ringtail habitat. The number of ringtails per unit area was determined by dividing the total number captured at a study site by the total area, giving what amounts to an "ecological" density (Odum 1971; Smith 1980).
To facilitate handling of ringtails, Tranvet 25[5] was used during the early stages of this study. The Tranvet was orally administered using an eyedropper. Dosages ranging from 0.16 ml. to 0.30 ml. were given to individuals weighing from 870 gm. to 1300 gm.
The effect of the Tranvet was not consistent. Hence, an alternative and more effective means of subduing the ringtails was sought. Ketamine hydrochloride,[6] a derivative of phencyclidine, was successfully used. Dosages approximating 0.01 ml. per 100 gm. of body weight were sufficient to sedate ringtails in 1-4 minutes for the 10–20 minutes necessary to tag, measure, weigh, and examine them. Recovery from the drug occurred 30–60 minutes after injection.
Each captured ringtail was weighed (to the nearest 10 gm.) in a burlap sack. Data on the following variables were taken: 1) sex; 2) lengths of tail, body, right hind foot, right ear-to-notch, and right ear-to-crown; 3) circumferences of neck and cranium (all measurements recorded in mm.); 4) pelage condition; and 5) body temperature. A numbered ear tag[7] was fixed to the medial proximal edge of the right ear. Animals were released at the point of capture, after recovery from the drug.
The vegetation on one of the study sites (Site V) was analyzed by the plotless pointquarter and quadrat methods (Cottan and Curtis 1956; Cox 1976). Motroni (1978, 1979) used these techniques to collect data on the composition of riparian vegetation at four of the study sites (Site I, II, III, IV). Overstory, midstory, and understory layers were distinguished (as described by Motroni 1978) and analyzed separately. The plotless point-quarter method was used to collect data on overstory and midstory vegetation. The quadrat method was used to evaluate the understory. In this study, the boundaries of the Central Valley correspond to those of "California Prairie" described by Küchler (1977), excluding that found within Monterey County.
Study Sites
Ringtail densities were determined at five study sites in riparian vegetation of the Sacramento Valley (fig. 1).
Study Site I
Preliminary trapping efforts were conducted at the Bobelaine Audubon Sanctuary, located on the Feather River near its confluence with the Bear River, approximately 3.2 km. (2.0 mi.) north of the town of Nicolaus, Sutter County, Califor-
[4] Tomahawk Live Trap Co., P.O. Box 323, Tomahawk, Wisconsin 54487.
[5] Propio-promazine hydrochloride, 100 mg./ml., Diamond Laboratories, Inc., Des Moines, Iowa.
[6] Vetelar, Parke-Davis & Co., Detroit, Michigan 48232.
[7] No. 1, monel metal, Jiffy wing bands from National Band and Tag Co., Newport, Kentucky.
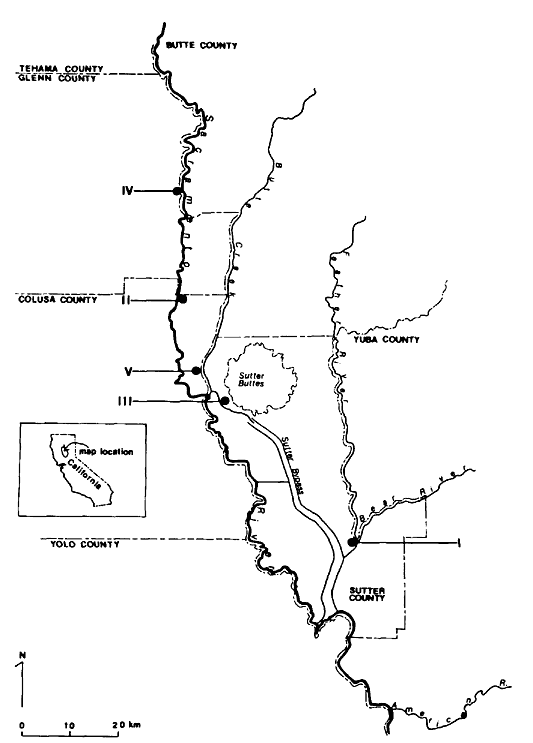
Figure l.
Ringtail study site locations in the Sacramento Valley, California.
nia (38° 56' 15" north, 121° 35' 45" west). The sanctuary is bordered on the east by the Feather River, on the west by walnut orchards, and on the north and south by riparian woodland. A slough traverses the western edge of the sanctuary and at one point widens into a 4.7 ha. (11.5 ac.) pond.
An extensive vegetation survey of the study site was conducted by Motroni (1979). Five major plant associations were recognized: 1) river grassland; 2) riparian shrub; 3) oak woodland; 4) cottonwood forest; and 5) riparian forest. It was within the last two plant associations and primarily in the riparian forest bordering the slough and pond that the majority of ringtails was livetrapped. These areas comprise approximately 69 ha. (172 ac.). Motroni (ibid .) found the overstory of the riparian forest to be dominated by cottonwood (Populusfremontii ). Other species present included box elder (Acer negundo ), valley oak (Quercuslobata ), and black walnut (Juglanshindsii ). The dense midstory was dominated by wild grape (Vitiscalifornica ), poison oak (Rhusdiversiloba ), Mexican tea (Chenopodium ambrosioides ). Other species present included blackberry (Rubusvitifolius ), coyote bush (Baccharispilularis var. consanguinea ), and wild rose (Rosacalifornica ). The understory was dominated by bermuda grass (Cynodondactylon ).
Study Site II
This 88-ha. (218-ac.) site is located on the Henry Womble property on the east bank of the Sacramento River 2.4 km. (1.5 mi.) south of Princeton, Colusa County, California (39° 22' 30" north, 122° 00' west). This is virtually a riparian island bordered by the Sacramento River and surrounded on three sides by agricultural fields. There are two small ponds, each less than 0.8 ha. (2 ac.), on the north side of the study site. An oxbow borders the east edge and curves west through the site where it eventually meets the Sacramento River. The vegetation has been described by Motroni (1978). Cottonwood was the dominant overstory tree, with willow (Salix sp.), valley oak, black walnut, and Oregon ash (Fraxinuslatifolia ) also present. Wild grape, blue elderberry (Sambucuscaerulea ), black walnut, and box elder formed a dense, often impenetrable midstory. Wild grape dominated the understory much the same as it did the midstory. Also abundant in this layer were blackberry and poison oak.
Study Site III
This 19-ha. (48-ac.) site, the Butte Slough State Wildlife Area, is located in Sutter County 26.4 km. (17 mi.) west of Yuba City (39° 9' north, 121° 53' west). Butte Slough forms the western boundary; agricultural land borders the site to the north and south, and a narrow band of oak woodland savannah was found along the east edge (beyond which is agricultural land). A 1.2-ha. (3-ac.) pond is located near the east edge of the study site.
This site differs markedly in vegetative composition and physiognomy from all other study sites. The riparian forest was a non-contiguous stand. Motroni (1978) found the overstory dominated by cottonwood with some willow and traces of valley oak. The relatively open midstory was dominated by Oregon ash. Buttonbush (Cephalanthusoccidentalis var. californicus ), valley oak, and box elder were also present in this layer. Poison oak, wild grape, and cocklebur (Xanthium strumarium var. canadense ) dominated the understory.
Study Site IV
This 76-ha. (187-ac.) site is on the west bank of the Sacramento River 6.9 km. (4.3 mi.) north of Glenn County, California (39° 35' north, 122º 00' west). It was formerly owned by Louis Heinrich, but now is a state wildlife area, and is bordered by the Sacramento River to the east and by agricultural land on three sides. A slough branches off of the river and borders the west side of the study area. Cottonwood and willow dominated the overstory, with box elder, black walnut, and sycamore (Platanus racemosa ) also present in this layer (ibid .).
Blue elderberry, willow, box elder, and black walnut combined to form a dense midstory, much like that found at Study Site II. Mugwort (Artemesiadouglasiana ), box elder seedings, wild cucumber (Marahfabaceus ), bed straw (Galiumaparine ), poison oak, black walnut seedlings, various grasses, wild grape, and blackberry formed the understory. Although not included in the vegetation survey conducted by Motroni (ibid .), approximately one-fourth of the study area was composed of a sparse stand of cottonwood with wild grape forming lianas similar to that described by early explorers of the Sacramento Valley.
Study Site V
This 82-ha. (202-ac.) site is 4.8 km. (3.0 mi.) northeast of Colusa, Colusa County, California (39° 16' north, 121° 57' west). It is adjacent to Butte Lodge Outing Duck Club and adjoins 0.2 km. of Butte Creek. A narrow slough runs the length of this riparian forest. Willow and valley oak dominated the overstory, with cottonwood, Oregon ash, and box elder contributing to form a dense canopy. Density of the midstory, dominated by poison oak, was variable. Oregon ash, box elder, buttonbush, and valley oak were also relatively abundant in this layer. Areas immediately adjacent to water were occupied by extensive stands of wild grape and willow forming a dense, nearly impenetrable midstory similar to Study Sites I, II, and IV. The closed canopy precluded much development of an understory. Poison oak was the dominant understory species, with blackberry, wild grape, mugwort, buttonbush, valley oak seedlings, and various grasses also present.
Results and Discussion
Ringtails were livetrapped or reported to exist at numerous locations in the Central Valley, including five of the nine sites sampled during this study (table 1; fig. 2, 3). Sightings ranged from the northernmost portion of the Valley near Red Bluff, Tehama County, to Stockton, San Joaquin County. These data represent a range extension, notwithstanding Naylor and Wilson (1956).
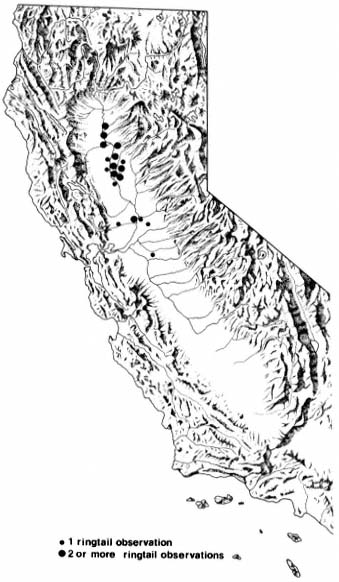
Figure 2.
Sites at which observations of ringtails have been
made within the Central Valley of California,
showing spatial relation to the rest of the state.
With few exceptions, ringtails were found to be associated with remnant stands of riparian forests bordering waterways such as the American River, Sacramento River, Feather River, Butte Creek, and Butte Slough. No ringtails were captured or reported from open, park-like stands of valley oak woodland.
Ringtails were also captured by Trapp in riparian vegetation in the Sutter Buttes, a small, isolated mountain range in the lower central portion of the Scramento Valley. Walt Anderson[8] reported that ringtails or their signs have been seen in other vegetation-types in the Sutter Buttes, including the blue oak woodland.
It is unlikely that ringtails have dispersed into the Valley since the statewide survey by Grinnell etal . (1937). In fact, evidence, apparently overlooked by Grinnell etal ., exists in the Museum of Vertebrate Zoology, University of California, Berkeley, from the 1930's which indicates ringtails were present at the Sutter Buttes (table 1, Sutter County). In their discussion of ringtail habitat preference, Grinnell etal . (ibid .) indicated that outside of the preferred "brushy or chaparral type" habitat, ringtails may also be ". . . found along streams (where) their range extends down to 500
[8] Walt Anderson. 1981. Biologist, Colusa, California. Personal communication.
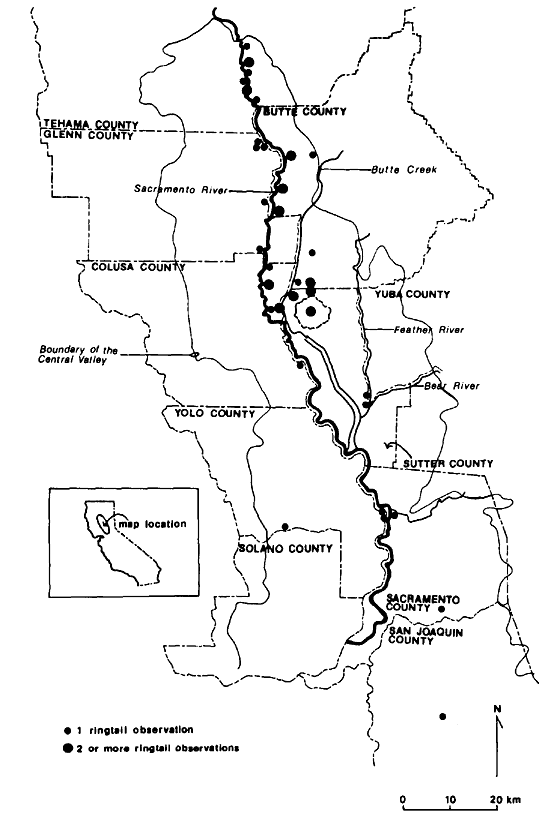
Figure 3.
Observation sites of ringtails in the Central
Valley of California, showing relation to
county boundaries and other local features.
ft. or to the mouths of canyons". Careful examination of the Grinnell range map (ibid .) revealed five ringtail sightings extending from near Red Bluff to the southern border of Tehama County. These sightings, which were just below 160 m. (500 ft.) and within the limit of California Prairie (Küchler 1977, i.e. the Central Valley), appeared to follow the Sacramento River. It is not clear why ringtails were not expected to occur in similar habitat below 160 m. It appears that ringtails were largely overlooked within the Central Valley and that there was simply a lack of effort by other investigators to document their presence prior to the current study.
The lack of sightings for the southern half of the Central Valley could be related to several factors. Historically, there was considerably less riparian vegetation here than in the northern Central Valley (ibid .; Roberts etal . 1977). A large portion of the remaining riparian vegetation has been eliminated in the path of agricultural expansion. Limited habitat availability, coupled with a less intensive survey effort by us in that area may explain why the range appears restricted to the northern portion of the Central Valley.
Trapping efforts undertaken to determine the abundance of ringtails in riparian forests of the Sacramento Valley produced reasonably consistent results where habitat features and trapping efforts were similar (table 2, 3). At study sites II, IV, and V densities of ringtails were 20.5, 17.1, and 19.5 individuals per 100 ha., respectively. Study sites I and II, where habitat features were different or where trapping effort was significantly less, exhibited densities of 11.4 and 10.5 ringtails per 100 ha.
At study site III, trap nights per ha. and trap nights per capture exceeded that of other sites (table 3); however, ringtail density at this site was lowest. There are probably a
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
variety of environmental factors involved in this lower value. This site, which borders Butte Slough, was a relatively narrow, broken stand of riparian forest with little of the grape or poison oak lianas so characteristic of the other sites. A comparison of the vegetation composition revealed a less diverse canopy and midstory. The canopy was composed almost exclusively of cottonwood. The relatively open midstory was dominated by Oregon ash.
Trapp (1972, 1978) documented ringtail anatomical and behavioral adaptations to climbing, as well as their ability to fully utilize all accessible aspects of the terrestrial environment. The ability to exploit the vertical aspect of their environment may allow ringtails to take advantage of a dense midstory, thereby increasing mobility about the forest. During times of winter flooding this could be especially important. A reduction, then, in a potentially critical aspect of their environment such as density of midstory vegetation might manifest itself in a smaller population size. This may partially explain variations in ringtail abundance.
Study sites II, IV, and V had similar ringtail densities and relatively diverse canopy and dense midstory vegetation layers. All three sites contained contiguous stands of riparian forest. Although the vegetation at study site I appeared to be physiognomically similar to that at study sites II, IV, and V, ringtail density was lower. This probably reflects a less intensive trapping effort (table 3).
There are few accounts in the literature on the abundance of ringtails and other procyonids. Grinnell etal . (1937) relied on the observations of W.H. Parkinson for density estimates. On 26 km2 (10 mi2 ) of land near Tollhouse, Fresno County, Parkinson trapped 31 ringtails within a season. Near the same area and over a 0.8-km (0.5-mi.) trail, Parkinson captured 13 ringtails. At 1,067 m. (3,500 ft.) on the western slope of Provo Mountain, near the Tuolumne River, within 0.65 km2 (0.25 mi2 ), he discovered five ringtail nests in blue oak trees. Each was occupied by a single ringtail. With information such as this, Grinnell etal . (ibid .) surmised that there was one ringtail per 13 km2 (5 mi2 ) throughout its range and in a few favored localities there were 2.3 per km2 (6 per mi2 ).
Taylor (1954) reported that J.D. Bankston, a trapper estimated ringtails at 3.9 per km2 (10 per mi2 ) in suitable broken country of the Edwards Plateau region in Texas.
Using home range data, collected with the use of radio-telemetry, Trapp (1978) estimated the density of ringtails in pinyon/juniper, blackbrush, and riparian vegetation of Zion Canyon, southwest Utah, to be 1.5–2.9 per km2 (3.8–7.6 per mi2 ).
Ringtail densities of 10.5–20.5 per 100 ha. (26.7–52.8 per mi2 ) documented during this study are the highest reported in the literature. Assuming that riparian woodland has higher productivity per unit area compared to chaparral and chaparral/woodland vegetations, the variation in ringtail density from shrub-dominated vegetationtypes to riparian woodland might be explained on the basis of this factor. A direct relationship
may also exist between ringtail density and physiognomic complexity (e.g., stratification) of the riparian plant community. Some evidence exists to support this contention from studies of the coati (Nasuanarica ), also a procyonid. The coati exploits arboreal aspects of its habitat (Davis 1960). Lanning (1976) reported densities of 1.2–2.0 coatis per 100 ha. in mixed evergreen woodland in and near Chiricahua National Monument, southeast Arizona. Densities of 42 and 26 coatis per 100 ha. have been estimated for tropical forests in Panama (Kaufmann 1962, cited by Lanning 1976). Lanning indicates that such differences in density may be attributed, in part, to ecological differences between the semiarid evergreen woodland and the moist tropical forests.
Acknowledgments
Special thanks are extended to Paul Laubacher, Randy Gray, Bob Motroni, Linda Heath, and particularly Dennis Messa for assistance in the field. Thanks to Gordon Gould, California Department of Fish and Game, for his cooperation and for arranging for the printing and mailing of the Furbearer Observation Questionnaire. Bob Motroni provided helpful suggestions of the techniques used for vegetational analysis. Larry Salata is gratefully acknowledged for critically reading and providing useful suggestions for this paper.
Literature Cited
Cottam, G., and J.T. Curtis. 1956. The use of distance measures in phytosociological sampling. Ecology 37:451–460.
Cox, G.W. 1976. Laboratory manual of general ecology. 232 p. Wm. C. Brown Co., Dubuque, Iowa.
Davis, W.B. 1960. The mammals of Texas. Texas Game and Fish Commission, Austin. Bulletin No. 41. 252 p.
Grinnell, J., J. Dixon, and J.M. Linsdale. 1937. Furbearing mammals of California. 2 vol., 777 p. University of California Press, Berkeley.
Hall, E.R. 1981. The mammals of North America. 2nd ed., 2 vol. 1181 p. John Wiley & Sons, New York, N.Y.
Hall, E.R., and K.R. Kelson. 1959. The mammals of North America. 2 vol., 1083 p. Ronald Press Co., New York, N.Y.
Ingles, L.G. 1965. Mammals of the Pacific states. 506 p. Stanford University Press, Stanford, Calif.
Kaufmann, J.H. 1962. Ecology and social behavior of the coati, Nasuanarica , on Barro Colorado. University of California Publ. Zool. 60:95–222 (cited by Lanning 1976).
Küchler, W.A. 1977. The map of the natural vegetation of California. p. 909. In : M.G. Barbour and J. Major (ed.). Terestrial vegetation of California. 1002 p. John Wiley & Sons, Inc. New York, N.Y.
Lanning, D.V. 1976. Density and movements of the coati in Arizona. J. Mamm. 57(3):609–611.
Long, C.A., and H.B. House. 1961. Bassariscusastutus in Wyoming. J. Mamm. 42(2):274–275.
Michny, F.J., D. Boos, and F. Wernette. 1975. Riparian habitats and avian densities along the Sacramento River. California Department of Fish and Game Administrative Report No. 75–1. 42 p.
Motroni, R.S. 1978. Sacramento Valley critical riparian habitat inventory. California Department of Fish and Game unpublished manuscript. 43 p.
Motroni, R.S. 1979. Avian density and composition of a riparian forest, Sacramento Valley, California. M.S. Thesis, California State University, Sacramento. 172 p.
Naylor, A.E., and G.W. Wilson. 1956. Unusual occurrence of the ring-tailed cat. Calif. Fish and Game 42(3):231.
Odum, E.P. 1971. Fundamentals of ecology. 3rd ed. 574 p. W.B. Saunders Co., Philadelphia, Penn.
Roberts, W.G., J.G. Howe, and J. Major. 1977. A survey of riparian forest flora and fauna. p. 3–19. In : A. Sands (ed.). Riparian forests in California: their ecology and conservation. 122 p. Institute of Ecology, University of California, Davis.
Schempf, P.F., and M. White. 1977. Status of six furbearer populations in the mountains of northern California. 52 p. USDA Forest Service Publications, California Region.
Seton, E.T. 1929. Lives of game animals. Vol. 2, Part I. Doubleday Doran & Co., Inc., Garden City, N.Y.
Smith, R.L. 1980. Ecology and field biology. 3rd ed. 835 p. Harper & Row Pub., New York, N.Y.
Stone T.B. 1976. Observations on furbearers within the riparian habitat of the upper Sacramento River. California Department of Fish and Game Memorandum Report. 12 p.
Taylor, W.P. 1954. Food habits and notes on life history of the ring-tailed cat in Texas. J. Mamm. 35(1):55–63.
Trapp, G.R. 1972. Some anatomical and behavioral adaptations of ringtails, Bassariscus astutus . J. Mamm. 53(3):549–557.
Trapp, G.R. 1978. Comparative behavioral ecology of the ringtail and gray fox in southwestern Utah. Carnivore 1(2):3–32.
Rare, Threatened and Endangered Invertebrates in California Riparian Systems[1]
Larry L. Eng[2]
Abstract.—Three California invertebrates dependent upon riparian systems have been listed as Rare, Threatened, or Endangered. These species, the California freshwater shrimp (Syncaris pacifica ), the valley longhorn beetle (Desmoceruscalifornicusdimorphus ), and the Trinity bristle snail (Monadeniasetosa ), represent three classes (two phyla) and occupy distinctly different habitats. Other species, not officially listed, are equally or perhaps more endangered; however, available information for official status determination is inadequate. The ultimate listing of all deserving invertebrates is an unlikely, if not impossible, goal given the dearth of data and the sheer number and diversity of species involved. Efforts should be directed at protecting and preserving ecosystems which are threatened rather than expended on individual endangered species which may occupy only a portion of the threatened ecosystem.
Introduction
Riparian systems support, either directly or indirectly, an abundance and diversity of wildlife (Sands 1978). Many of the species of animals and plants recognized as Rare, Threatened, or Endangered by state and federal agencies are directly or indirectly dependent upon riparian areas for their survival (ibid; Hirsch and Segelquist 1978). The primary reason for the listing of most of these species (and the primary cause for their status) is the substantial loss or degradation of their habitats. Hirsch and Segelquist (1978) estimated that about 70–90% of natural riparian areas have been destroyed or extensively altered.
Recognition of the magnitude of the loss and concern over the continuing assaults on the remnant riparian areas has resulted in several symposia designed to increase awareness not only of the threats to riparian systems, but also of their importance and value. In these symposia and in other forums, emphasis has been placed on plants and vertebrate animals. The invertebrate species in riparian systems have received little consideration. To a degree, this reflects the orientation of the participants, but it also reflects the dearth of information available on invertebrate species dependent on riparian areas.
But even with the emphasis on vertebrates, Bury et al . (1980) expressed concern that "the great bulk of vertebrate species [the nongame species] are not receiving the share of attention that they deserve as interesting and important members of most natural communities." This lack of attention is even more glaring for the invertebrate component of riparian systems.
In this paper I will discuss the officially listed Rare, Threatened, and Endangered invertebrates dependent upon riparian areas in California, the threats to their continued existence, and the need for a different approach in obtaining protection for endangered and rare species.
Threatened Riparian Invertebrates
Riparian ecosystems are composed of a wide variety of environments and microenvironments, some of which support invertebrate species with very specialized habitat requirements. These specialists, which are unable to compensate or substitute for lost environments are experiencing the most immediate threats as remnant riparian areas continue to shrink.
Within California three species of invertebrates, representing two phyla and three classes, have been officially designated as Endangered, Rare, or Threatened. These species are the California freshwater shrimp (Syncarispacifica ),
[1] Paper presented at the California Riparian Systems Conference. [University of California, Davis, September 17–19, 1981].
[2] Larry L. Eng is Invertebrate Biologist, Endangered Species Program, California Department of Fish and Game, Rancho Cordova, Calif.
the Trinity bristle snail (Monadeniasetosa ), and the valley elderberry longhorn beetle (Desmocerus californicusdimorphus ). These species, each of which is dependent upon a markedly different type of habitat, are discussed in more detail below.
California Freshwater Shrimp
The California freshwater shrimp (Syncaris pacifica ) (fig. 1), a small freshwater shrimp which lives in lowland streams in Marin, Sonoma, and Napa counties, was designated an Endangered species by the California Fish and Game Commission in 1980 (Eng 1981). It is the only surviving member of the genus Syncaris . Its congener S . pasadenae was extirpated by urban development in southern California. Although an aquatic species, S . pacifica is dependent upon riparian vegetation for food and shelter. During fall and winter months, S . pacifica lives among submerged exposed roots beneath undercut banks, where it is protected from downstream displacement during heavy runoff from winter rains. The tree roots not only provide cover for the shrimp, but also reinforce the streambank enabling the undercuts to persist.
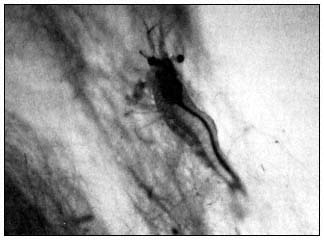
Figure l.
The California freshwater shrimp (Syncaris pacifica ).
The principal riparian plants comprising the shrimps' habitat are alders (Alnus ), willows (Salix ), blackberries (Rubus ), sedges, and ferns. The leafy branches of these plants grow into the water during spring and summer, providing cover and food for the shrimp. The shade provided by the larger trees reduces solar heating of the stream. This shading is especially important during droughts when the shrimp are confined to remnant pools. During spring the shrimp move out from the undercut banks and live on the submerged leafy branches of streamside vegetation. They also utilize the exposed hair-like adventitious roots of alders growing along the stream margin. The submerged leaves and filamentous roots collect detrital material and serve as substrates for bacteria and other decomposers, thus providing a food source for the omnivorous shrimp. Submerged and decomposing leaves may also be consumed by the shrimp.
Habitat degradation has resulted in the extirpation of this species from at least five streams (Hedgpeth 1975). Some longtime residents of the area report that the shrimp once occurred in virtually every stream in the three county area; however, we have records of their existence, historically, in only 10 streams (Eng 1981). Continuing urban and residential development pose threats to streamside vegetation, especially in Sonoma County, one of the fastest-growing counties in the state. Hedgpeth (1968) reported that S . pacifica was extirpated in Santa Rosa Creek by an urban improvement project in Santa Rosa. Livestock grazing continues to be a problem locally, causing loss of riparian vegetation and collapse of streambanks. Construction of summer dams and artificial beaches for recreational purposes has destroyed substantial amounts of shrimp habitat in some streams. Vineyard development has also resulted in the loss of substantial amounts of riparian vegetation. In many cases, the vineyards extend to the stream margin.
Valley Elderberry Longhorn Beetle
The valley elderberry longhorn beetle (Desmocerus californicusdimorphus ) (fig. 2) is a rare longhorn beetle, known from only a few localities in the lower Sacramento and upper San Joaquin valleys in California. These beetles are restricted to riparian areas, where the larvae are obligate stem and root borers of elderberry (Sambucus sp.). The adults feed on the foliage of the same plant.
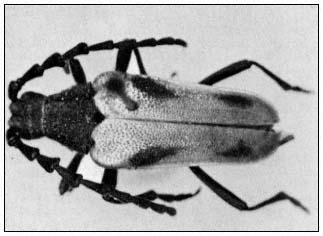
Figure 2.
The valley elderberry longhorn beetle ( Desmocerus
californicus dimorphus ). (Photo by F.G. Andrews.)
The habitat of this longhorn beetle occurs in areas with relatively high human population density and intensive agriculture. Much of the Central Valley riparian vegetation, of which elderberry is a conspicuous component, has al-
ready been lost, primarily to urban and agricultural development. Extensive loss of essential habitat for this rare species during historical times and the continuing threats to its remaining habitat were major forces in the decision of the US Department of the Interior to list this species as Threatened in 1980. Loss of elderberry groves continues as agricultural and urban development expand. Construction of industrial parks, bicycle trails, and parking lots all take their toll on the beetle's shrinking habitat. Obviously, some critical density of elderberry plants is required for the maintenance of the beetle's populations; however, studies to determine that necessary density have yet to be undertaken.
Trinity Bristle Snail
The Trinity bristle snail (Monadeniasetosa ) (fig. 3) is a terrestrial snail, living in the riparian zone along several small, primary streams in the Trinity River drainage in Trinity County (Roth and Eng 1980). Adults of this nocturnal species are most commonly found among leaf litter on the forest floor, although they have been observed several feet up in the branches of deciduous trees.[3] Very young snails are apparently dependent upon standing dead trees, spending the first several months of their existence living beneath the loose bark. Trinity bristle snails are active during the moist months of the year, feeding on fungi and decomposing leaves.

Figure 3.
The Trinity bristle snail (Monadenia setosa ).
The Trinity bristle snail occurs only in the presence of a hardwood understory including bigleaf maple (Acer macrophyllum ), dogwood (Cornus sp.), and California hazel (Coryluscornuta var. californica ). In the lower stream reaches, white alder (Alnus rhombifolia ), California black oak (Quercus kellogii ) and tanbark-oak (Lithocarpus densiflora ) are sometimes interspersed with the above species. The decaying leaf litter from these trees apparently constitutes a major food source for the snail (Roth and Eng 1980). On the dryer upper slopes M . setosa is replaced by its cogener M . churchi ; it is also absent from lightly shaded areas of exposed hillsides.
The Trinity bristle snail is restricted to sparsely populated, mountainous Trinity County. Much of its habitat is on national forest land. Because of its restricted habitat and very limited range, the California Fish and Game Commission has designated it a Rare species under California law. Threats to this species are logging activities which would encroach on the riparian zone. Fires, road construction, erosion, and removal of standing trees utilized by the juvenile snails would all have substantial deleterious effects on the snail's habitat. Other potential threats come from gold-mining operations and the development of small hydroelectric projects. The USDA Forest Service has responded to the presence of this rare snail and has developed an interim management plan for it (Armijó 1979).
Discussion
Only three invertebrates directly dependent upon riparian systems in California have been legally recognized as Rare, Threatened or Endangered species by the state or federal government. However, these animals come from very different habitats, represent three different classes and two different phyla, and undoubtedly constitute only the tip of the iceberg of threatened invertebrate species. Official recognition of these three species does not mean that they are the only California riparian invertebrates that are threatened. What it means is that enough data and support existed to enable an official determination of their status to be made. A number of other invertebrates dependent upon riparian systems (Roth 1972; Donahue 1975; Hunt and DeMartini 1979; Murphy 1979) have been proposed for federal listing, but the USDI Fish and Wildlife Service considered available information inadequate to make a final determination. Data for most other invertebrate species are even more limited.
We know that many invertebrates are restricted to riparian systems and many others utilize these areas facultatively or as "migration" corridors (Merritt and Cummins 1978; Shapiro 1974). The importance of riparian systems to certain groups was illustrated by Shapiro (1974), who found that 85% of the species comprising the Sacramento Valley butterfly fauna occur along the lower American River.
The riparian systems of California include a wide variety of environments. Many invertebrates inhabiting these areas have highly specialized habitat requirements; others are geographically very localized. Although the continuing loss of riparian systems has resulted in extensive habi-
[3] Roth. Personal communication.
tat loss for many species, it is an especially urgent threat to those species that are highly specialized and geographically restricted.
The lack of information on invertebrates makes it difficult to demonstrate that the existence of a species is threatened (the minimum requirement for federal listing) or even that it ". . . exists in such small numbers throughout its range that it may be endangered if its environment worsens" (the minimum requirement for state listing).[4] All this is complicated by a general ignorance of and/or prejudice against invertebrates in general and insects in particular by politicians and the general public. The possibility of a backlash against listing any invertebrate is very real.
The universal threat to riparian systems seems sufficient justification to list those many invertebrate species dependent upon them, especially the rare and highly specialized forms, as Rare, Threatened, or Endangered. The large number of species in this category and the dearth of information on most of them makes the listing of each qualified species unlikely, if not impossible, under present standards of administrative review.
In most cases, a species becomes officially listed as Threatened or Endangered when it can be shown that a substantial loss of its habitat has reduced its abundance to the point where its future survival is threatened. Because the environment which provides this habitat is shared by a variety of other species (in the community sense), some of which are restricted to only portions of that environment, the few listed species should be considered indicators of threatened environments or ecosystems. Planning and management should be directed toward activities which emphasize the protection and preservation of the entire ecosystem which is threatened, rather than focused on a single endangered species which may represent only a portion of the threatened ecosystem. The ultimate success in protecting all species belonging to threatened riparian ecosystems requires the development of a means of protecting these systems without necessitating an official listing of each threatened or endangered component.
Acknowledgments
I would like to thank Stephen J. Nicola, California Department of Fish and Game, for providing helpful comments on a draft of this paper.
Literature Cited
Armijó, P. 1979. Monadeniasetosa (California northern river snail): interim species management plan. 24 p. USDA Forest Service, Shasta-Trinity National Forest, Redding, Calif.
Bury, R.B., H.W. Campbell, and N.J. Scott. 1980. Role and importance of nongame wildlife. Trans. 45th North Amer. Wildl. Nat. Res. Conf. 1980: 197–207.
Donahue, J.P. 1975. A report on the 24 species of California butterflies being considered for placement on the federal lists of endangered or threatened species. 58 p. California Department of Food and Agriculture, Sacramento. Unpublished manuscript.
Eng, L.L. 1981. Distribution, life history, and status of the California freshwater shrimp, Syncarispacifica (Holmes). California Department of Fish and Game, Inland Fish. Endangered Species Program Pub. 81–1, Sacramento. 27 p.
Hedgpeth, J.W. 1968. The atyid shrimp of the genus Syncaris in California. Int. Revue Ges. Hydrobiol. 53: 511–524.
Hedgpeth, J.W. 1975. California fresh and brackish water shrimps, with special reference to the present status of Syncarispacifica (Holmes). USDI Fish and Wildlife Service, Office of Endangered Species, Contract 14-16-0008-841, Final Report, Washington, D.C. 27 p.
Hirsch, A., and C.A. Segelquist. 1978. Protection and management of riparian ecosystems: activities and views of the US Fish and Wildlife Service. p. 344–352. In : R. R. Johnson and J. F. McCormick (tech. coord.). Strategies for protection and management of floodplain wetlands and other riparian ecosystems. USDA Forest Service GTR-WO-12, Washington, D.C. 410 p.
Hunt, H., and J.D. DeMartini. 1979. Administrative study of the Karok Indian snail, Vespericolakarakorum , Talmadge 1962. 20 p. USDA Forest Service, Shasta-Trinity National Forest, Redding, Calif.
Merritt, R.W., and K.W. Cummins. 1978. An introduction to the aquatic insects of North America. 441 p. Kendall/Hunt Publishing Company, Dubuque, Iowa.
Murphy, D.D. 1979. Butterfly survey: Inyo National Forest. USDA Forest Service, Inyo National Forest, Report RFQ R5-04-78-008 (43-91W2-8-747). 37 p. Unpublished manuscript.
[4] California Fish and Game Code Section 2051B.
Roth, B. 1972. Rare and endangered land mollusks in California. California Department of Fish and Game, Inland Fish. Admin. Rep. 72-10, Sacramento. 21 p.
Roth, B. and L.L. Eng. 1980. Distribution, ecology, and reproductive anatomy of a rare land snail, Monadeniasetosa Talmadge. Calif. Fish and Game 66:4–16.
Sands, A. 1978. Public involvement in riparian habitat protection: a California case history. p. 215–227. In : R. R. Johnson and J. F. McCormick (tech. coord.). Strategies for protection and management of floodplain wetlands and other riparian ecosystems. USDA Forest Service GTR-WO-12, Washington, D.C. 410 p.
Shapiro, A. M. 1974. The butterfly fauna of the Sacramento Valley, California. J. Res. Lepidoptera 13:73–82, 115–122, 137–140.
Gray Fox Temporal and Spatial Activity in a Riparian/Agricultural Zone in California's Central Valley[1]
Donald L. Hallberg and Gene R. Trapp[2]
Abstract.—Gray fox (Canis [Urocyon ] cinereoargenteus )[3] temporal activity is quantitatively described from 1,094 radio telemetry fixes obtained from two male and two female subjects studied on Putah Creek, near Davis (Yolo County), California, from March through July 1973. The subjects were found to exhibit similar non-random temporal activity. Significant (p £ 0.05) increases in diurnal activity occurred one to two hours prior to sunset. Minimum activity began mid-morning and reached a low in late afternoon. Regardless of time, diurnal travel rates were conspicuously lower than nocturnal travel rates. All subjects occupied essentially the same area and had similar home range size (129 ha.). They spent 75.7% of the nocturnal and 91.6% of the diurnal period in riparian zones and the rest of the time on agricultural lands.
Introduction
The extensive range of the gray fox (Canis [Urocyon]cinereoargenteus ), both in Latin America and the contiguous United States, as shown by Hall and Kelson (1959), suggests an ability to adapt readily to widely varying environmental situations. Hence, it is of value to learn how behavior varies in specific parts of its wide range.
Gray fox temporal activity is poorly understood. In 1972, an extensive gray fox literature review was conducted by Trapp and Hallberg (1974). At that time, the number of published references to fox circadian activity were limited to less than a half-dozen (Seton 1929; Grinnell etal . 1937; Taylor 1943; Gander 1966). With the exception of Taylor's (1943) work, little more than passing remarks were made concerning circadian activity.
In Texas, Taylor (ibid .) quantitatively described the activities of four captive animals during one 22-hour period. His conclusions generally agreed with those of Grinnell et al . (1937) in California and Seton (1929), who suggested crepuscular and nocturnal activity is most common.
Howver, Gander (1966), observed that gray foxes visited his southern California feeding station at all hours of the day and night, implying that diurnal as well as nocturnal activity is not unusual. A more recent and somewhat more quantitative investigation conducted from 1967 to 1969 in southwest Utah by Trapp (1978) concluded that: "Foxes, though active mostly at night, also forage diurnally and crepuscularly to a lesser, but important, extent."
Only limited information was available concerning gray fox home ranges. Richards and Hine (1953) in Wisconsin reported home ranges of 13–310 ha., while Lord (1961) in northern Florida estimated gray fox home ranges to be about 770 ha. Using telemetry techniques in southwest Utah, Trapp (1978) determined the mean home range to be 107 ha. It is not clear if the variation in home range size was due to differences in population densities (Trapp and Hallberg 1974), variations in habitat productivity, sampling errors, or other factors.
The present project's objective was to expand upon the temporal aspects of gray fox
[1] Paper presented at the California Riparian Systems Conference. [University of California, Davis, September 17–19, 1981.]
[2] Donald L. Hallberg is Associate Data Processing Analyst, California Department of Fish and Game, Sacramento. Gene R. Trapp is Associate Professor of Biological Sciences, California State University, Sacramento.
[3] New genus as suggested by Van Gelder (1977, 1978).
natural history by quantitatively describing circadian activity in relation to habitat utilization. Activity data were secondarily expected to provide information concerning home range and intraspecific interactions.
This paper is based on thesis research undertaken in 1973 (Hallberg 1974).
Description of Study Area
The study area (fig. 1) is located at 38° 32' N and 121° 41' W, 6.4 km. (4 mi.) southeast of Davis, California. The site is situated on private agricultural land immediately adjacent to the Yolo Bypass. Putah Creek's south fork bisects the study area longitudinally for approximately 4.8 km. (3 mi.) and is paralleled by flood control levees. Putah Creek meanders between the levees and in most cases is bordered by a 0.16 to 0.32-km. (0.1- to 0.2-mi.) wide agricultural belt. During the study period, principal crops associated with the belt included varieties of tomatoes, beans, melons, and wheat.
The area study contained approximately 88 ha. (218 ac.) of stream and riparian zone consisting of approximately 36% tree and shrub cover, 46% open grassland, and 18% covered by water. Fremont cottonwood (Populusfremontii ), black walnut (Juglanshindsii ), and large willow (Salixlaevigata ) were plentiful, bordering the creek channel. Less abundant were blue elderberry (Sambucusmexicana ), box elder (Acer negundo ), tamarix (Tamarisk gallica ) and valley oak (Quercuslobata ). Dense sandbar willow (Salixhindsiana ) thickets were common in low, damp, sandy areas.
Extensive milk thistle (Silybummarianum ) stands, some as large as 0.2 ha. (0.5 ac.), were common in early spring. They were usually associated with disturbed areas around the perimeter of open grassy areas. By July, most of the areas of annual grass were overgrown by yellow star thistle (Centaureasolstitialis ).
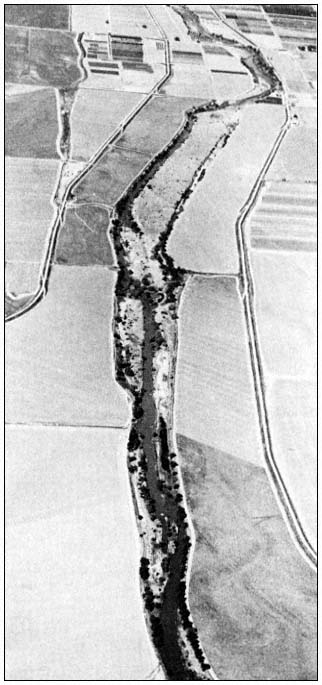
Figure l.
Gray fox study area along Putah Creek, southeast of Davis,
California, as seen from an altitude of approximately 914 m.
(3,000 ft.), looking west. Stream and riparian ststem are
flanked by agricultural fields and flood control levees. All
telemetry fixes were taken from gravel roads on the
levees paralleling the narrow agricultural belts.
Methods and Materials
Field data were obtained primarily by radiotelemetric monitoring (Hallberg etal . 1974) and secondarily by direct observation.
Trapping
Trapping and processing techniques were similar to those employed by Trapp (1978) to capture gray foxes and ringtails (Bassariscusastutus ) in Zion National Park, Utah. Nineteen collapsible Tomahawk double-doored livetraps (23 x 23 x 66 cm.) were placed in the field during March 1973 and left for the project's duration. Each was positioned in or near a well-defined trail, camouflaged with surrounding debris, and baited with raisins whenever it was necessary to capture animals.
Telemetry
Four gray foxes, two males and two females, were captured. Each was fitted with a radio-
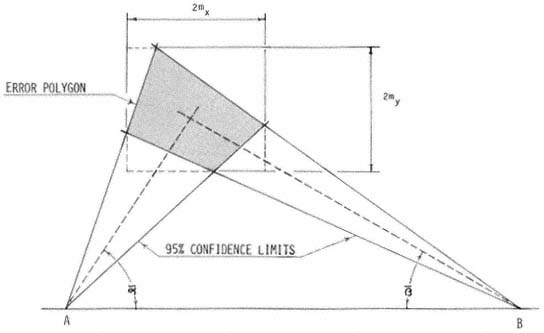
Figure 2.
Error polygons were computed by first calculating confidence limits (95%) for
bearing means (
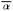
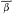
intersection. Each fix was then expressed as the intersection of bearing means
(
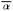
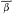
polygon. The dimensions 2mx and 2my represent the 95% confidence range
associated with the fix along the X and Y axes respectively.
[4] Source listings of all Fortran IV computer programs are included in Hallberg (1974).
telemetry collar and monitored from 25 March through 27 July 1973. The effective telemetry range varied with atmospheric conditions, but the system usually performed well when within 0.16–0.8 km. (0.1–0.5 mi.) of the subject. Under ideal conditions signals were received from distances exceeding 1.6 km. (1 mi.).
Diurnal resting places were located with a three-element, hand-held yagi antenna and Davidson Model W portable receiver. All triangulation data were collected using a vehicle-mounted broadside array, consisting of two vertically polarized 3-element yagi antennas. The array pivoted from a television mast which also supported a Suunto compass from a projecting arm (Hallberg etal . 1974).
Each subject's location with respect to time was determined from field bearings, recorded to the nearest 0.5° of direction, being determined in the following manner:
1) two to five bearings were made in rapid succession and recorded from a predetermined station in the subject's vicinity;
2) the telemetry vehicle was quickly driven to an adjacent station where a second series of bearings was taken.
The entire procedure required two to five minutes to complete and was similar to the technique employed by Ables (1969) and Trapp (1978). During field observations, an attempt was made to locate two or three subjects every 30 minutes (mean = 34 minutes; range = 4 to 282 minutes; n = 1,094). It was not feasible to locate all subjects every 30 minutes because they were frequently too far apart.
Data Processing
All field bearings were later converted into fix coordinates; their associated error polygons (fig. 2)(Heezen and Tester 1967) were computed in relation to a single (x,y) coordinate system by computer.[4] The error polygons were used to test the significance of the distance between fixes. When successive fixes were shown to be significantly different (ca. p = 0.05), the subject was judged to have moved and therefore determined to be active. When significant movement was not demonstrated, the subject was considered inactive, even though undetectable activity may have occurred. For active subjects, mean travelrate index values were computed from elapsed time and distance measurements. Rates were considered to be only indices, since there was no reason to suspect foxes moved at a constant rate between
fixes or that they traveled in straight lines. All data were related to either sunrise or sunset to correct for variations in day length during the study.
Results and Discussion
Radio telemetry tracking allowed positive identification of subjects and their location (fix precision: 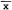
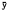
No subject was observed to scratch, pull, or otherwise react to the telemetry collar once it was attached. Upon release subjects seemed to have little difficulty negotiating dense vegetation; after recapture several months later no skin chafing was evident. Based upon these observations it was assumed that the telemetry collar had little or no effect upon the subject wearing it.
Temporal Behavior
Statistical independence of subject circadian activity was tested by contingency analysis (Ostel 1963; Adler and Roessler 1972). No substantial evidence was found to suggest that subjects exhibited different circadian activity probabilities, and male:female activity probabilities did not significantly differ (p = 0.05).
The apparent similarity of subject activity probabilities was used to justify lumping all fox data. This in turn provided a larger sample for statistical inference. The pooled data were examined by testing a series of hypotheses. The first assumed the probability of activity to be random with respect to time; that is, the probability of activity equals the probability of inactivity for any given hour. Chi square analysis resulted in the rejection of this hypothesis (p = 0.05) for 19 hours of the day (fig. 3). The five hours where activity was random were the first, second, and sixth hours after sunrise, the first hour prior to sunset, and the seventh hour after sunset. Failure to reject the null hypothesis for the seventh hour after sunset was considered to be due to an inadequate sample (n = 2). Three of the four remaining one-hour intervals appeared random because these intermediate periods occurred while significant increases or decreases (p = 0.001) in activity states were in progress. The remaining period, six hours after sunrise, could not be explained on the basis of these data.
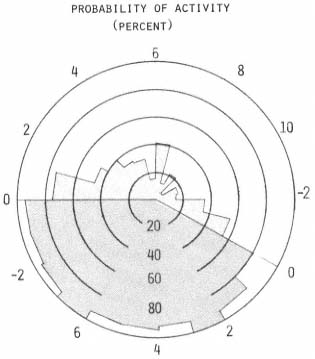
Figure 3.
The probability of activity [(active fixes/total fixes)
x 100] is shown for each hour. Hourly polar divisions
relate either to sunrise or sunset. The diurnal period
is shaded lightly with respect to the nocturnal period.
In general, it appeared that gray fox activity periods occurred in non-random fashion, foxes being significantly (p = 0.001) less active during diurnal periods than nocturnal. Statistically significant changes (p = 0.01) in activity appeared during the first two hours after sunrise and the first and third hours prior to sunset. Minimum activity observed was during mid-afternoon, which was significantly less (p = 0.001) than that observed in late morning.
Gray fox activity probabilities seem to be similar to those observed by Storm (1965) for five red foxes (Vulpes fulva ) in Illinois. Storm stated that the daily journeys began as early as two hours before the night and usually continued throughout most of the night; sometimes they continued as late as four hours after dawn. Rymills (1979) found that gray fox activity at Point Reyes National Seashore, California, generally began just before dark and continued until dawn.
Travel Rates
An animal's rate of movement probably depends upon many factors, such as its activity, travel conditions, the weather, and presence of other animals (Sanderson 1966). Since the mean rates, computed for each hour, were influenced greatly when extremes were encountered, no statistical inference was made. However, several interesting trends were apparent. Rates were relatively constant during diurnal periods and considerably lower than nocturnal values (fig. 4). This suggests that the type of activity occurring during diurnal periods was similar for each hour, regardless of activity probability. In contrast, nocturnal rates averaged about twice the diurnal values and exhibited two peak periods, while activity probabilities remained consistently above 80%.
Figure 4.
The mean travel rate in feet per minute
was calculated for each hour. See figure
3 for description of polar graph.
Rate Indices
Neither activity probabilities nor mean travel rates seemed to reflect properly the intuitive impression of gray fox temporal movement developed while monitoring the subjects. However, by computing a rate index statistic (rate index is the product of activity probability and respective mean travel rate), that took into account both the probability of activity and mean travel rate, a more representative graph was constructed (fig. 5). The nocturnal period was clearly the most active time for each subject. Animals did not move long distances during diurnal periods, except perhaps just before dusk.
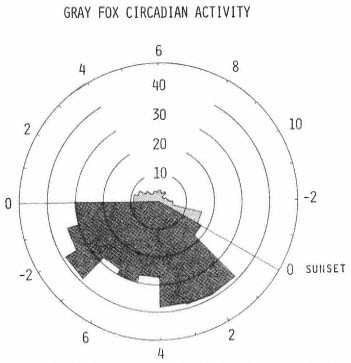
Figure 5.
An index representing the product of the hourly activity
probability from figure 3 and the respective mean travel
rate from figure 4 was calculated for each hour. See
figure 3 for description of polar graph.
Field observations suggest that three categories of activity may exist; short moves, meanders, and purposeful traverses. Extrapolation from these assumptions, based upon the available literature, suggests that a gray fox is likely to leave its diurnal resting site shortly before sunset and move only short distances while investigating the immediate area before beginning a purposeful traverse toward a foraging area some distance away. Perhaps this was the type of movement observed by Richards and Hine (1953) in Wisconsin when they reported that gray foxes frequently followed fence rows or well-defined trails. Upon arriving at the foraging area, more time was spent investigating thickets and crevices.
During meandering movements, many abrupt turns and reversals in direction occurred, as was also observed by Trapp (1978) in southwest Utah.
Two or three hours before sunrise a subject often made a second purposeful traverse toward the area in which it would spend the day, before extensive movement ceased at sunrise. Only short distances were traversed during diurnal periods as, perhaps, a fox moved from one resting site to another to take advantage of varying sun-shade conditions or pursued a potential meal.
Seasonal Activity
Spring and summer diurnal activity probabilities could not be demonstrated as similar by contingency analysis (p = 0.05). Spring diurnal activity was essentially non-existent from mid-morning through mid-afternoon. During the summer, substantially more activity occurred for the same period. No tangible evidence was found that explained this dichotomy. However, it seems reasonable that diurnal activity would be reduced for secretive carnivores during that portion of the year when only sparse vegetative cover was available.
The study area provided little cover during March and April, since the annual and perennial grasses were short and deciduous trees and shrubs
did not provide an extensive canopy. The only heavy groundcover available was an occasional brush pile or milk thistle stand. Similarly, certain spring and summer nocturnal activity probabilities could not be demonstrated as being the same, although the differences were not as dramatic as the diurnal differences. This may have been because the concealment afforded by darkness reduced the cover's influence. These assumptions are not supported in the literature since Wood (1954) and Richards and Hine (1953) stated that gray foxes are most active in the fall and winter, presumably when cover is minimal.
Further support for the "availability of cover" hypothesis was shown by the location of diurnal resting sites. In spring, it was not uncommon for a gray fox to return to the same resting area each day for several days in a row. Once the deciduous canopy began to develop, it was more common for subjects to rest in different areas each day. On 26 occasions, actual diurnal resting sites were visually located with the aid of a portable yagi antenna and receiver. In early spring, these sites were always in a dense stand of milk thistle. By late spring, after the vegetative canopy began to develop subjects were found in dense sandbar willow thickets or occasionally in a brush pile or under a tamarisk. None of the sites, except one in a milk thistle stand, appeared to be subterranean.
Reference to computer-drawn maps for each observation period suggested that subjects daily traversed more of their respective home ranges during June and July than in earlier months.
Home Range
Individual gray fox home ranges (fig. 6) were delineated by minimum polygons (Hayne 1949) drawn around the perimeter of fixes from each subject using Ables' (1969) "atypical habitat elimination method." The subjects were found to have a mean home range of 129 ha. (range = 106–172, n = 4). Fuller (1978), working approximately 6.4 km. (4 mi.) west on Putah Creek in more diverse habitat, calculated four home ranges (30, 132, 142, and 185 ha.) which gives a mean home range size of 122 ha. For eight gray foxes in Zion National Park, Utah, Trapp (1978) calculated a similar mean home range (107 ha.). However, Rymills (1979) computed a smaller mean home range (50 ha.) for three gray foxes at Point Reyes National Seashore, California.
Home ranges for all subjects closely coincided with the riparian zone and adjacent agricultural belts. The importance of the riparian zone as habitat seems evident, since 96.1% of the inactive observations were made within it. Probably less than three hours in any 24-hour period were spent in the bordering agricultural areas, and much of this time undoubtedly was spent travelling the dirt roads that separated riparian and agricultural areas (cf . Richards and Hine 1953; Wood 1954).
The 6-m. high flood-control levees apparently had minimal effect upon lateral movement outside the riparian zone, since the band of lateral activity remained relatively consistent even in the southeastern portion of the study area where no levee existed (fig. 1).
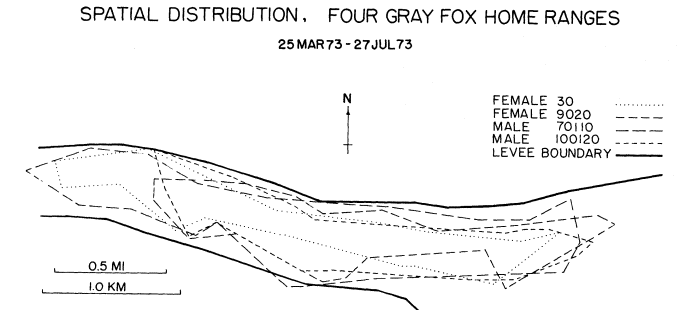
Figure 6.
Individual home ranges of four gray foxes on the Putah Creek study area, Davis, California.
Each home range was determined from computer analysis of radio-telemetry data. The
levee boundary can be compared with the study area photograph in figure 1.
The home ranges of all four subjects closely coincided (fig. 6). The activity centers (Hayne 1949) were also nearly coincident, suggesting that these animals were not territorial toward each other.
No subject limited its inactive periods to a specific region within the riparian zone, and only 5.9% of the fixes for different subjects appeared to overlap. This suggests that no apparent regional territoriality existed between subjects, although certain inter-individual distances were maintained.
Reducing the number of inactive areas by only counting those locations in which eight or more fixes were recorded reduced the number of inactive sites from 101 to 23 (fig. 7). These 23 sites reflected 60.9% of the inactive observations. Each subject still appeared to rest in various areas throughout the riparian zone, but never was observed at a resting site that had been frequented by another subject.
The apparent solitary diurnal behavior of the subjects did not change appreciably during nocturnal periods. None of the subjects were observed to travel together, although they may have met for brief periods. The frequency of such meetings could not be determined since the subjects often followed what appeared to be erratic paths with many reversals in direction. Presumably, erratic and solitary behavior would allow maximum exploitation of a rather limited range by several gray foxes.
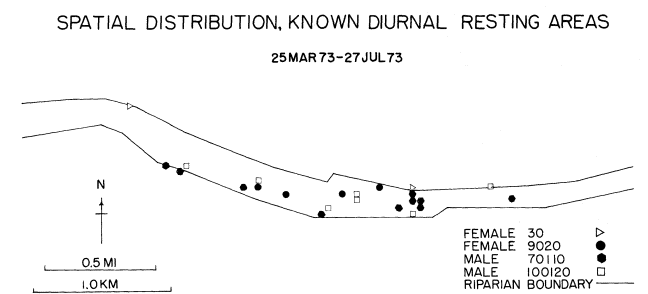
Figure 7.
The spatial distribution of known diurnal gray fox resting
areas as determined from computer analysis of radio-telemetry
data taken on the Putah Creek study area, Davis, California.
Conclusions
In a habitat of riparian/agricultural land on Putah Creek, near Davis, California, four gray foxes (two males and two females) were found to exhibit similar temporal and spatial habits. Some temporal movement was observed during all 24 hours of the day. Activity increases began as early as two hours prior to dusk and peaked about three hours after sunset. The probability of activity exceeded 80% for the remainder of the nocturnal period. Although activity probabilities showed a significant reduction two hours following sunrise, minimum activity did not occur until afternoon.
Travel rates suggested that subjects made only local moves during diurnal periods and did not begin longer traverses until an hour after sunset. Although nocturnal travel was extensive, peak travel rates were observed one hour after sunset and again three hours before sunrise. These peak periods seemed to occur as subjects were leaving diurnal resting areas or when returning to them.
Individual gray foxes normally rested in different locations each day, once vegetative cover became abundant in late spring. After dark, activity paths appeared erratic, showing many twists and reversals in direction, particularly during the summer. No differences in circadian rhythms were observed during spring and summer, although the degree of spring activity was reduced. The lower activity probabilities and travel rates in the spring were attributed to sparse vegetative cover.
All subjects appeared to have nearly identical home ranges, since the geographical area and centers of activity were similar. The narrow riparian zone appeared to be the primary influence upon the home range's shape.
The apparent similarity of subject activity was not attributed directly to positive intraspecific co-actions since each subject appeared to exhibit solitary resting and travel habits.
Literature Cited
Ables, E. 1969. Home range studies of red foxes (Vulpesvulpes ). J. Mammal. 50(1):108–119.
Adler, H.L., and E.B. Roessler. 1972. Introduction to probability and statistics. 373 p. W.H. Freeman and Co., San Francisco, Calif.
Fuller, T.K. 1978. Variable home-range sizes of female gray foxes. J. Mammal. 59(2):446–449.
Gander, F.F. 1966. Friendly foxes. Pacific Discovery 19(1):28–31.
Grinnell, J., J. Dixon, and J.M. Linsdale. 1937. Fur-bearing mammals of California. 2 volumes, 777 p. University of California Press, Berkeley, Calif.
Hall, E.R., and K.R. Kelson. 1959. The mammals of North America. 2 volumes, 1,083 p. The Ronald Press, New York, N.Y.
Hallberg, D., F. Janza, and G. Trapp. 1974. A vehicle-mounted directional antenna system for biotelemetry monitoring. Calif. Fish and Game 60(4):172–177.
Hallberg, D.L. 1974. A contribution toward the better understanding of gray fox (Urocyon cinereoargenteus ) temporal and spatial natural history. M.S. Thesis, California State University, Sacramento. 285 p.
Hayne, D.W. 1949. Calculation of size of home range. J. Mammal. 30(1):1–18.
Heezen, K.L., and J.R. Tester. 1967. Evaluation of radio-tracking by triangulation with special reference to deer movements. J. Wildl. Mgmt. 31(1):124–141.
Lord, R.D. 1961. A population study of the gray fox. Amer. Midl. Nat. 66(1):87–109.
Ostle, B. 1963. Statistics in research. 585 p. Iowa State University Press, Iowa.
Richards, S.H., and R.L. Hine. 1953. Wisconsin fox populations. Wisconsin Conservation Department, Tech. Wildl. Bull. No. 6. 78 p.
Rymills, E.M. 1979. Movements and food habitats of gray fox, Urocyoncinereoargenteus , in Point Reyes National Seashore. M.A. Thesis, San Francisco State University, San Francisco, Calif. 131 p.
Sanderson, G.C. 1966. The study of mammal movements: a review. J. Wildl. Mgmt. 30(1): 215–235.
Seton, E.T. 1929. Lives of game animals. Vol. 1, Part 2. pp. 340–640. Doubleday, Doran and Co., Inc., Garden City, N.Y.
Storm, G.L. 1965. Movements and activities of foxes as determined by radio tracking. J. Wildl. Mgmt. 29(1):1–13.
Taylor, W.P. 1943. The grey fox in captivity. Texas Game and Fish 1(10):12–13, 90).
Trapp, G.R. 1978. Comparative behavioral ecology of the ringtail and gray fox in southwestern Utah. Carnivore 1(2):3–32.
Trapp, G.R., and D.L. Hallberg. 1974. Ecology of the gray fox (Urocyoncinereoargenteus : a review. p. 164–178. In : M.W. Fox, ed. The wild canids. 508 p. Van Nostrand Reinhold Co., N.Y.
Van Gelder, R.G. 1977. Mammalian hybrids and generic limits. Amer. Mus. Novitates 2635: 1–25.
Van Gelder, R.T., 1978. A review of canid classification. Amer. Mus. Novitates 2646:1–10.
Wood, J.E. 1954. Investigations of fox populations and sylvatic rabies in the Southeast. p. 131–139. In : Trans. 19th North American Wildl. Conf.
Fish Slough
a Case Study in Management of a Desert Wetland System[1]
E. Philip Pister and Joanne H. Kerbavaz[2]
Abstract.—Fish Slough is a remnant of a once-widespread, shallow aquatic/riparian wetland in the arid Owens Valley (Inyo and Mono counties, California). Fish Slough supports a variety of rare species, including the endangered Owens pupfish. Successes and failures of management efforts at Fish Slough should hold lessons for management of other endangered species and natural areas.
Introduction and Background
The first recorded observation of the Owens pupfish (CyprinodonradiosusMiller ) (fig. 1) occurred in 1859 when Captain J.W. Davidson of the US Army described vast numbers of pupfish throughout the wetland areas of the Owens Valley (Inyo and Mono counties, California). So abundant were the small cyprinodont fishes that local Indians would seine them with woven baskets and dry them in the sun for winter food (Wilke and Lawton 1976).
Figure l.
Owens pupfish (Cyprinodon radiosus Miller). From top
to bottom: adult female, adult male, subadult female.
Pupfish numbers remained high until at least 1916, when Clarence H. Kennedy, a student from Cornell University, observed large schools of pupfish in the numerous sloughs and swamps between Laws and Bishop (Kennedy 1916). Their time was short, for already severe changes were being effected which would bring about an enormous reduction in the once-abundant wetlands that support this fish. Numbers would be reduced to a point where, when described as a species in 1948, the Owens pupfish would be thought to be extinct (Miller 1948).
The investigations of Carl L. Hubbs and Robert R. Miller during the 1930s and 1940s revealed that because of reduction in surface water supplies, the habitat (and the fish) was progressively becoming reduced in extent. Its remnant habitat was being reduced and confined to the "type locality," that location from which the species was originally collected for official taxonomic description. This locality was described by Miller (ibid .) as: "the northwestern feeder spring of Fish Slough, about 10 miles north of Bishop, California." Today this location is a portion of the Owens Valley Native Fish Sanctuary.
Probably the major factor involved in such severe habitat reduction was the development and export of Owens Valley water to supply burgeoning populations in Los Angeles (Heinly 1910). Then, in later years dams were constructed to retain waters that, during and since the Pleistocene epoch, had periodically covered the Owens River floodplain and created ideal habitat for native fish populations. Nearly as damaging as water development and export, and occurring during this
[1] Paper presented at the California Riparian Systems Conference. [University of California, Davis, September 17–19, 1981.]
[2] E. Philip Pister is Fishery Biologist, California Department of Fish and Game, Bishop, Calif. Joanne H. Kerbavaz is Environmental Planner, California Department of Transportation, Bishop, Calif.
same general time period, was the introduction of predaceous gamefishes and other exotic species, including the western mosquitofish (Gambusiaaffinis ), which preyed upon and competed with a constantly decreasing pupfish population (Pister 1974).
As habitat was reduced and populations of competing and predaceous fishes grew, the pupfish was gradually pushed to its final toehold in the marshlands of Fish Slough (Miller and Pister 1971). It is significant to note that among four native fishes in the Owens River system, two are listed as endangered (Owens pupfish and Owens chub [Gila bicolorsnyderi ]) and one is threatened (Owens dace [Rhinichthysosculus ssp.]). Only the Owens sucker (Catostomusfumeiventris ) remains in substantial numbers (Pister 1981).
Preservation and Management
Following rediscovery of the Owens pupfish in 1964, thought began to be directed toward its management. Inventories of all fish populations began to be worked into general management plans of the California Department of Fish and Game (DFG), which had up to that time been so completely dedicated to gamefish management that any species not possessing adipose fins or spiny rays became an immediate candidate for eradication (Pister 1976). Suggestions for preserving such things as snails and plants met with derision. Problems of preserving all life forms at that time were more political than biological (Pister 1979).
Changes in the natural character of the Owens Valley continued through the next decade, largely manifested in the loss of spring ecosystems (and their associated flora and fauna) through increased groundwater extraction. This gradual change was accompanied during the 1960s by at least two instances during which pupfish populations in natural habitats thought to be secure were nearly lost.
So on June 26, 1967, when Carl Hubbs, Bob Miller, and Phil Pister met at Fish Slough to consider the possibility of creating refugia for the native fishes of the Owens Valley, their thinking went well beyond that. It was becoming disturbingly clear that the remaining aquatic wetland in Fish Slough was the only area in the entire Owens Valley retaining even a semblance of the magnificent ecosystem that existed before the coming of Europeans. More than fish refugia were needed. Aquifers supplying the springs had to be protected, and private inholdings had to be acquired to minimize further impact on the Fish Slough ecosystem.
The first priority was preservation of the Owens pupfish. Refugia were constructed at two locations in Fish Slough in the early 1970s. Designed to prevent the invasion of introduced predatory fishes that abound in Fish Slough, the refugia attempted to recreate the conditions under which the native Owens Valley fishes evolved (Miller and Pister 1971). The refugia have been successful in protecting the native fishes, and in 1980 the Owens Valley Native Fish Sanctuary (the first refuge constructed) was expanded to enhance Owens pupfish habitat (fig. 2). Current status and recovery efforts for the Owens pupfish are summarized by Courtois and Tippetts (1979).
Public resistance to the creation of a native fish sanctuary is not widespread, but certainly exists. Fences have been cut, signs torn down, and largemouth bass (Micropterus salmoides ) frequently (and illegally) planted into the refuge area. Such actions, although frustrating, only serve to strengthen our resolve to protect the entire ecosystem under a comprehensive management plan.
The Fish Slough Refuge
Fish Slough, with its permanent water sources, is an aquatic anomaly in an arid valley and a remnant of a once-widespread shallow wetland. As such it supports a variety of rare species, including the pupfish, an undescribed snail, and at least six rare plants, as well as a dense concentration of cultural sites. It is not just the species and the cultural resources that are rare, it is the wetland with its aquatic and riparian systems.
Ownership boundaries in Fish Slough do not correspond with natural boundaries. As in most of the Owens Valley, much of the actual riparian land is owned by the Los Angeles Department of Water and Power (LADWP). The USDI Bureau of Land Management (BLM) administers land with one of the slough springs and manages most of the surrounding dry shadscale scrublands. There were two private inholdings in Fish Slough. DFG acquired a 64.8-ha. (160-ac.) parcel at the mouth of the Slough in the mid-1970s. The other parcel, 81.8 ha. (202 ac.) about 1.6 km. (1 mi.) southwest of the main slough springs, remains in private hands.
Of the riparian landowners, only DFG has the luxury of managing its lands exclusively for the benefit of the pupfish and the desert riparian system. The LADWP is concerned with developing and maintaining water supplies for export; this concern can conflict with needs for water for instream and riparian uses. BLM labors under a multiple-use mandate and must balance competing human needs and resource values.
Beyond the landowners, additional state and federal agencies and organizations have an interest in Fish Slough. Foremost among them has been the University of California Natural Land and Water Reserves System (NLWRS). The NLWRS, as part of an effort to preserve unique natural systems throughout the state for teaching and research, attempted to purchase the 81.8-ha.

Figure 2.
Prime Owens pupfish habitat, looking west across Fish Slough to the Sierra Nevada.
private inholding during the 1970s. These attempts were not successful, but the NLWRS Systemwide Advisory Committee and staff continue to support efforts to obtain the inholding and establish a reserve.
To reconcile varying interests and develop an effective management program, representatives of the three landowning agencies and the NLWRS met in 1975 and drafted a cooperative management agreement. The agreement recognized the needs and responsibilities of the various agencies, as well as the need to manage Fish Slough as an ecological unit.
The cooperative agreement has allowed the continuing efforts of DFG to protect and enhance pupfish populations. But the native fish refugia and the desert riparian system cannot be considered secure while there are threats of development and changes in water supply for the slough.
In July 1979, the owners of the 81.8-ha. private inholding filed Tentative Tract Map 37-23 Zack with Mono County. They proposed creating the new housing subdivision of Panorama Tuff Estates, with 49 parcels ranging from 1.2 to 4.3 ha. (3 to 10.6 ac.) in size.
The immediate threat to the integrity of Fish Slough galvanized support for a refuge among the landowning agencies and other interested parties. They convinced the Mono County Board of Supervisors to defer action on the subdivision map and concentrated efforts on the acquisition of the parcel.
The present landowners object to increased government land ownership in the Owens Valley, an area where governmental agencies own an estimated 98% of the land. The owners refused to sell their land, but agreed to exchange the parcel in Fish Slough for developable land somewhere else.
BLM began negotiations to decide on parcels to exchange. Even when both sides agree that an exchange will be mutually beneficial, the exchange process is long and arduous. The process includes a series of agreements, appraisals, and approvals and requires the involvement of several levels of BLM hierarchy.
In the case of Fish Slough, the exchange requires, in addition, the proverbial "Act of Congress." As is the case on much of the public land in the Owens Valley, the parcel selected by the private landowners had been withdrawn for watershed protection for the City of Los Angeles. This withdrawal must be shifted to the parcel in Fish Slough. The necessary legislation, HR 2475, is pending.[3]
Interagency efforts have focused on the prevention of permanent damage to the slough. With the acquisition of the final private inholding, the involved agencies and groups can work towards the creation and management of a reserve to protect all of Fish Slough.
Lessons Drawn from the Fish Slough Case
The unique cooperative management efforts at Fish Slough to save an endangered species and a threatened ecosystem offer the opportunity to assess successes and failures and to draw lessons for the future.
One of the primary lessons taught by this case study is the reaffirmation of one of Murphy's Laws, that anything you try to fix will take longer and cost more than you thought. Attempts to remedy the effects of water diversion, habitat destruction, and unwise species introductions have required a significant investment of time and resources. This seemingly clear-cut case, the effort to preserve a universally recognized significant natural area, has taken almost 20 years to bring towards completion.
This case demonstrates the potential value of vigorous interagency cooperation. For this project, the pathways were primarily informal ones, built from common concern for protection of threatened resources. It would be difficult to plan in advance for cooperation like that needed for this project; however, it is imperative that land management agencies retain the flexibility to seek innovative, cooperative solutions.
Work to acquire the private inholding shows that we must develop and improve alternatives to fee acquisition in California. Money for land purchases is limited, and some landowners, unwilling to sell, may be interested in other alternatives.
The BLM land exchange process can be a valuable tool, especially in California's desert areas, to both eliminate inholdings and provide land more suited to development in less sensitive areas. The process is time-consuming and cumbersome, however, and many landowners have neither the time nor the patience to work with it.
Many authors have explored the alternatives to fee acquisition for the protection of natural resources (Hoose 1981). We must make it a priority to bring the best of these alternatives to fruition. We can start by making existing options, such as land exchanges, more workable.
Conclusion
Never before have the critical relationships between habitat integrity and species existence been brought so sharply into focus as during the 1970s, the decade of the endangered species preservation movement. The near extinction of the Owens pupfish and elimination of its desert wetland is just one example of a situation that is being repeated throughout the world, as natural resources succumb to short-sighted drives for economic development. The forces that reduced the pupfish populations and habitat from abundance to virtual disappearance in only three decades warrant sober and critical reflection.
Literature Cited
Courtois, Louis A., and William Tippetts. 1979. Status of the Owens pupfish, Cyprinodon radiosus (Miller), in California. Inland Fisheries Endangered Species Program Special Publication 79-3, California Department of Fish and Game, Sacramento, Calif. 31 p.
Heinly, Burt A. 1910. Carrying water through a desert. National Geographic 21(7):568–596.
Hoose, Phil. 1981. Building an ark: tools for the preservation of natural diversity. 217 p. Island Press, Covelo, Calif.
Kennedy, C.H. 1916. A possible enemy of the mosquito. Calif. Fish and Game 2:179–182.
[3] Editor note: Although HR 2475 was unopposed, the political process is unpredictable and laborious at best. HR 2475 finally passed on 19 December, 1982, just a few hours before Congress adjourned. Final details of the land exchange are in the process of completion, and no further obstacles are anticipated.
Miller, R.R. 1948. The cyprinodont fishes of the Death Valley system of eastern California and southwestern Nevada. Miscellaneous Publications of the Museum of Zoology, University of Michigan 68:1–155.
Miller, R.R., and E.P. Pister. 1971. Management of the Owens pupfish, Cyprinodonradiosus , in Mono County, California. Transactions of the American Fisheries Society 100:531–540.
Pister, E.P. 1974. Desert fishes and their habitats. Transactions of the American fisheries Society 103:531–540.
Pister, E.P. 1976. A rationale for the management of nongame fish and wildlife. Fisheries 1:11–14.
Pister, E.P. 1979. Endangered species: costs and benefits. In : The endangered species: a symposium. Great Basin Naturalist Memoir 3:151–158.
Pister, E.P. 1981. The conservation of desert fishes. p. 411–445. In : R.J. Naiman and D.L. Soltz (ed.). Fishes in North American deserts. R. J. Naiman and D. L. Soltz (ed.). 552 p. John Wiley and Sons, Inc., New York, N.Y.
Wilke, P.J., and H.W. Lawton. 1976. The expedition of Captain J. W. Davidson to the Owens Valley in 1859. 55 p. Ballena Press, Socorro, N.M.
Geographical Ecology of the Sacramento Valley Riparian Butterfly Fauna[1]
Arthur M. Shapiro[2]
Abstract.—The Sacramento Valley butterfly fauna is depauperate and strikingly uniform in the riparian corridor from Redding to the Sacramento-San Joaquin Delta. There are few obvious pre-American relicts and only one taxonomically recognized endemic, Battusphilenor hirsuta . Historical reasons for these conditions are discussed along with aspects of the biology of characteristic riparian species.
Introduction
The Sacramento Valley has the same reputation among Lepidopterists as among weekend recreation-seekers: a hot, dry, flat, uninteresting place between the coast and the Sierra Nevada that should be traversed as quickly and painlessly as possible. To some extent the Lepidopterological reputation, at least, is deserved.
Before 1968 no faunistic or ecological treatment of the Valley butterflies had appeared. In that year Opler and Langston (1968) published a faunistic analysis for Contra Costa County which included part of the Sacramento-San Joaquin Delta, and with it a very significant proportion of the Valley fauna. The entire butterfly fauna of the Valley consists of 65 species, of which only about 50 can be considered permanent residents under present-day conditions (Shapiro 1974, 1975). How rich or poor a fauna is this? It would seem rich to an Englishman; the British Isles, with 243,500 km2 (94,000 mi2 ) and infinitely more topographic and vegetational diversity, have about the same number of species as 19,400 km2 (7500 mi2 ) of Sacramento Valley (Ford 1975). On the other hand, by temperate North American standards this is clearly a poor fauna. A disturbed tidal marsh and adjacent waste ground covering some 104 km2 (40 mi2 ) near Philadelphia boast a fauna of 73 species (Shapiro 1970). Staten Island, New York, has 104 species in 180 km2 (70 mi2 ) (Shapiro and Shapiro 1973). Closer to home, Gates and Mix Canyons on the east slope of the Vaca Hills, Inner Coast Range, Solano County, California, have as many species as the entire Sacramento Valley (Shapiro unpublished). Almost any montane locality in northern California has more. The Trinity Alps and Mount Eddy have about 112 species (Shapiro etal . 1981); in the Sierra Nevada, Donner Pass (2,130 m. (7,000 ft.)) has over 100 species in about 31 km2 (12 mi2 ), giving it one of the richest butterfly faunas in the North Temperate Zone (Emmel and Emmel 1962; Shapiro unpublished).
Apart from species numbers, a fauna may also be examined by taxonomic composition. An "unbalanced" fauna is one in which one or a few taxonomic groups provide most of the species. When the Valley fauna is compared to others in California, it seems relatively balanced—there is a slight deficit of Lycaenidae, which tend to be specialists, and a corresponding surplus of Hesperiidae, which may be relative generalists (though their ecologies are mostly unstudied).
The reasons for local and regional differences in faunal richness and composition are partly historical and partly ecological.
In Miocene and Pliocene times much of the present Sacramento Valley was a large, shallow inland sea. As noted by Ornduff (1974), one consequence of the emergence of the Valley floor as a habitat for terrestrial plants was the evolution of new and distinctive taxa, mainly ephemeral annuals: "The surrounding upland areas had been occupied by plants for a much longer period of time than the Central Valley and support evolutionarily older plant species and plant communities". The evolution of distinctive plants and communities on the Valley floor was apparently not mirrored in its butterflies.
The pre-American Valley supported three principal community-types: bunchgrass/valley oak savanna, tule/cattail marsh, and riparian forest (Thompson 1961; Sculley 1973). Although the early explorers have left vivid accounts of northern California scenery and wildlife, our knowledge of the butterfly fauna of the pristine Valley is virtually non-existent. A great many
[1] Paper presented at the California Riparian Systems Conference. [University of California, Davis, September 17–19, 1981].
[2] Arthur M. Shapiro is Professor of Zoology and Lecturer in Entomology, University of California, Davis.
common California butterflies were described in Europe by J.B.A. de Boisduval, who received them from a Frenchman, Pierre J. Lorquin, who had come during the Gold Rush of 1849. Lorquin's Admiral, (Limenitislorquini ) a common riparian butterfly, is named after him. Nearly all we know of his travels is from the sketchy outline provided in de Boisduval's monograph (1869). He may have followed the Sacramento River north through the Valley to its headwaters (F. Martin Brown, inlitt .). De Boisduval says he "explored first all the environs of San Francisco, then the banks of the Sacramento and the Feather, . . . made trips into the Sierra Nevada range, even to the forests of the interior, braving the tooth of the bear and the fangs of the rattlesnake." Valley localities are mentioned in the text; for example Pierisprotodice is cited as "common enough in the Sacramento [district]".
Lorquin also furnished California butterflies to Felder in Austria and to a Philadelphia entomologist, Tryon Reakirt, who may have described a now-extinct riparian population of the Pierisnapi complex from near Stockton (see below). None of the early works gives a good picture of the Valley fauna as a fauna; almost nothing of an ecological nature was published on California butterflies before Tilden's (1959) landmark paper on Tioga Pass! We do know that no species was described from the Valley only to become extinct later, as happened in the San Francisco Bay Area. Whether this reflects a true absence of Valley endemics or only a failure to collect there is another matter. I imagine there was inadequate collecting—of a very limited fauna!
It seems apriori unlikely that the adaptive radiation of ephemeral annuals would have been paralleled in the butterflies. The timing of vernal annual life cycles is largely dependent on rainfall, which is notoriously unreliable and unpredictable. The normal evolutionary response of butterflies to these conditions is long-distance migration (Larsen 1976), as is seen in the Painted Lady (Vanessacardui ) in both the Old World and the New, and in a more disorganized way, in the lowland Orange Sulphur (Coliaseurytheme ), Checkered White (Pierisprotodice ), and others.
The surviving vernal-pool communities in the Sacramento Valley have their specialist bees, but no butterflies. The only grassland butterfly associated with a native annual is the Large Marble (Euchloeausonides ), which has a facultatively bivoltine race we infer was originally associated with the mustard, Thelypodiumlasiophyllum , but is now "domesticated" on weedy European Brassica . Even here, the Valley population—though phenotypically distinctive—has not been recognized taxonomically, and belongs to a Holarctic complex with vernal phenology in the Old World as well as the New.
The most striking, and rather surprising, absence in the Valley grassland fauna is that of a set of specialist Satyridae or Hesperiidae associated with the native bunchgrasses. The near-extermination of these grasses leaves little hope of finding relicts of a (totally hypothetical) pre-American fauna. Only the endemic California Ringlet (Coenonymphatullia california ), occurs today in disturbed grassland throughout the state, west of the Sierra-Cascade axis. Most of the Hesperiid fauna is weedy, associated with introduced grasses. At least one species, Lerodeaeufala , seems to be a recent introduction (since the 1940s). Hesperia juba , otherwise montane, occurs at low densities in the Bay Area and in the Delta.
Equally surprising is the lack of a distinctive fauna in the marshlands. They are older than the grasslands, basically the remnants of the old shallow sea, and better preserved. We have only the Yuma Skipper (Ochlodesyuma ), which feeds on a native strain of the common reed (Phragmitescommunis ), and is still numerous in the Delta. There are relict marsh skippers in the montane part of northern California, but they are rare and perhaps in decline. Overall, the marsh fauna in California is poor when compared to those in the East.
The riparian butterfly fauna is the best preserved in the Valley. Although we cannot be absolutely confident that nothing has been lost, the uniformity of the fauna throughout the region suggests that we are seeing most of it. Indeed, it might almost be said that the present Sacramento Valley butterfly fauna is a riparian fauna—albeit one that has expanded into irrigated agricultural and especially urban environments. The remainder of this paper surveys its derivation, distribution, composition, and prospects.
The Riparian Fauna Today
Sources
As California dried out over several million years, and the present Mediterranean climate became established, many plant species requiring summer moisture contracted their ranges to the banks of the major watercourses. This emerging riparian vegetation included woody taxa, ultimately derived from Axelrod's Arcto-Tertiary Geoflora, which otherwise could not have survived in the absence of summer rain (Raven and Axelrod 1978).
Precisely the same thing seems to have happened to the butterfly fauna. A sizeable proportion of the Valley fauna is of Arcto-Tertiary origin, with strong affinities to the Palearctic. This element is prominent in the riparian faunas. In most lowland California habitats butterfly emergences are concentrated in spring, coinciding with the simultaneous occurrence of good flight weather and lush vegetation. Most species are univoltine and have a summer dormancy, or diapause. This applies not only to Madro-Tertiary derivatives but Arcto-Tertiary ones as well, which may be multivoltine (e.g.,
Pierisnapi ) or early-summer univoltine (Satyrium spp., Glaucopsychelygdamus ) in more humid climates. Outside the riparian zone there are few multivoltine species, and those that occur are migratory or at least extremely dispersive.
Continuous breeding in summer requires continuous availability of host plants in suitable condition and adult food sources through the long rainless months. Comparing curves representing the number of species flying throughout the year at Valley and foothill locations, the difference in seasonal pattern is striking (fig. 1). It is due to the preponderance of multivoltine species in the riparian zone. These butterflies have no option of summer dormancy, though they do diapause overwinter. They have in effect been sheltered from the summer drought and have never evolved special physiological adaptations to cope with it. They are still doing what their forebearers did in a climate with summer rain.
Figure l.
Number of species flying in the Vaca Hills and adjacent
Sacramento Valley during the 1972 season. The spring
peak in the hills is due to univoltine species, while most
Valley floor species are multivoltine. In the summer, annuals
are dried and dead. Perennials and woody plants have
ceased to add new growth. Thus both adult nectar and
larval food sources are unavailable in the Vaca Hills.
The preadapted character of this riparian fauna contrasts interestingly with the butterfly fauna of another region subjected historically to long-term drying: the Patagonian steppe. Patagonia has been deteriorating climatically since the Tertiary (Menendez 1972; Petriella 1972; Volkheimer 1971). The large rivers coming down from the Andes and crossing the vast treeless plateaus of Patagonia are fringed with a narrow band of riparian forest, but there is essentially no butterfly fauna there. Overall, the Patagonian butterfly fauna is far more depauperate and taxonomically unbalanced than that of the Sacramento Valley. The great majority of species are Satyrids that live not in the moist river bottoms but on the steppe proper, feeding on the bunchgrasses.
The historical basis for this apparent anomaly is straightforward. Given the past and present geography of the region, where would a riparian fauna be recruited from? Only the cool, humid Tertiary Nothofagus forests of the west slope of the Andes come to mind. But this is a very poor analogue of our Arcto-Tertiary sources. Arcto-Tertiary vegetation covered a vast area in the Northern Hemisphere and was extremely diverse. At that time the Nothofagus forests were already highly insular and much less diverse. Their extreme geographic isolation would have dictated an impoverished butterfly fauna even if the wet, cloudy climate had not. At the same time, an endemic butterfly fauna had differentiated in the treeless puna of the high Andes, and was able to colonize the cool-arid Patagonian steppe with little trouble. Even today, most of the shared taxa are undifferentiated even at the subspecies level. Clearly, the preponderance of riparian butterflies in the Sacramento Valley fauna does not represent a phenomenon inevitable in arid or semi-arid regions.
Although most of the Valley fauna seems to be of Arcto-Tertiary origin, two conspicuous species are not: Atlides halesus and Battusphilenorhirsuta . Atlides belongs to a purely Neotropical group. B . philenor has its closest relatives in tropical America but belongs to an old, pan-tropical group of Aristolochia -feeding Papilionidae best developed in southeastern Asia and the Australian region. Both B . philenor and its host plant, Aristolochiacalifornica may be relicts of Axelrod's Neotropical-Tertiary Geoflora, which is extinct at the level of woody plants. Alternately, they and A . halesus might have entered North America quite late in the "Great American Interchange" (Marshall 1981), as Anaea andria , Euptoietaclaudia , Eurema mexicana , and some other butterflies found in the eastern and southwestern United States almost certainly did. My own suspicion is that B . philenor , at least, is older.
Distribution
There are no endemic species in the butterfly fauna of the Sacramento Valley, and only one endemic subspecies (Battusphilenorhirsuta ). The riparian butterflies are largely widespread, generalized mesic species. Like the grassland and marsh faunas, they show little evidence of evolutionary activity. The fauna is extremely uniform from the head of the Valley to the Delta; the species lists for Turtle Bay (Redding) and Discovery Park (Sacramento) are nearly congruent. Nor is there north-south clinal variation or phenotypic differentiation, despite the increase in continentality in the climate of the north Valley.
These facts suggest genetic continuity in the riparian corridors, which have been fragmen-
ted only in recent historical time. Even in their present condition, these corridors are still probably adequate to permit the larger, more mobile species to maintain genetic continuity. Many riparian butterflies are excellent dispersers and are often seen as singletons many miles across hostile habitat from their nearest breeding colonies. B . philenor and Phyciodescampestris are good examples. Papiliorutulus has been documented as dispersing 8 km. (5 mi.).[3]
The question of whether these riparian corridors provided continuity across the Valley floor, conecting Coast Range and Sierran foothill populations, remains open. Most species are not phenotypically differentiated in the two foothill systems, and many of them occur in the isolated Sutter Buttes, even though there is no east-west continuity seen today (e.g., Papilio multicaudatus , Battusphilenor , Polygoniasatyrus , Vanessaatalanta ). Shapiro (1977) tells the strange story of Pieris napi castoria , described by Reakirt from Lorquin material possibly collected at or near the Valley town of Castoria (now French Camp, near Stockton); it is not known on the Valley floor today. The Chalcedon Checkerspot (Euphydryaschalcedona ) is a riparian/canyon species found in both sets of foothills which does not breed in the Valley today. It is phenotypically differentiated in both larval and adult characters between the two ranges, and has not been collected in the Sutter Buttes. Lorquin's Admiral shows weak phenotypic differentiation in northern California. Valley floor and Sierra populations are less orange below than those of the Coast Range, which resemble Trinity-Siskiyou area residents. Some of this variation may be under environmental control.
Some Distinctive Riparian Butterflies
Surveying the biology of some 50 taxa is beyond the scope of this paper. Readers desiring more complete documentation should consult Shapiro (1974, 1975). The following material presents information on the most distinctive components of the Sacramento Valley fauna.
Battus Philenor Hirsuta
The Hairy Pipevine Swallowtail, the Valley's only taxonomically recognized endemic entity, is also the most characteristic Valley and foothill riparian butterfly. It occurs everywhere that its sole host plant, Aristolochiacalifornica occurs. The species B . philenor occurs from Baja California and the Arizona desert to the mid-Atlantic coast. This subspecies, though weakly differentiated phenotypically, is unique in being completely disjunct from the rest of the species range. It is also unique in its population biology, sustaining extremely high densities in its larger colonies near Chico and in eastern Sacramento County. Its northern limit is near Dunsmuir, Siskiyou County. Except for an isolated record at North Bend, Oregon (Dornfeld 1980), it is completely restricted to the Sacramento Valley and adjacent canyons, and a few sites in the Bay Area.
The life-history of B . philenorhirsuta is of great ecological and evolutionary interest. Basically it is multiple-brooded, but part of the pupae in each generation go into diapause. Most of these do not hatch until the following spring, but a few emerge at irregular intervals the same season. The proportion diapausing, and the strength of the diapause, may be functions of the weather[4] rather than daylength, which determines these things in most mid-latitude butterflies.
The adaptive significance of this becomes apparent once the host relations of the butterfly are examined. Although large larvae can and do feed on mature Aristolochia foliage, young ones must begin feeding on the tender shoot tips, and it is here that the eggs are laid. In wet years the plants may put on some new growth all summer, and the butterfly can continue to breed. In very dry years breeding may become impossible as early as late June. The ratio of dormant to nondormant pupae in the very large spring brood thus represents a gamble on the condition of the plants a few weeks hence. The March–April flight is a mixture of adults from all the broods completed in the previous year. We are trying to learn how good this swallowtail is at predicting the condition of its host, and allocating its reproductive effort in different years.
Phyciodes Campestris
Not all Sacramento Valley populations of the Field Crescent occur in riparian forests; most are at the edges of such forests or of marshes. Its distribution largely coincides with that of its host plant, Asterchilensis . Aster is a prominent plant in mesic oldfield successions, but is rather rare and local in much of lowland California. Probably many colonies of both plant and butterfly remain undiscovered. In the southern Sacramento Valley (exclusive of the Delta, where it is relatively general), about six colonies are known. One of these (Willow Slough, north of Davis, Yolo County) has been under observation for 10 years. P . campestris is triple-brooded in the Valley and often very abundant where found. It overwinters as a larva, which can somehow survive being flooded for several weeks in winter.
Valley campestris show a distinctive summer phenotype not found elsewhere in the range, but we do not know if other populations would display it if reared under Valley conditions. The nominate subspecies with which our populations are lumped occurs from mesic sites in southern California (rare) north to Arctic Alaska. It also occurs east of the Sierra, as at
[3] L. Smith, personal communication.
[4] S.R. Sims, personal communication.
Mono Lake. Intervening between Great Basin and Sacramento Valley populations is a paler-colored entity, montana , endemic to montane and subalpine Sierran meadows. Typical campestris occurs in the same habitats in the Trinity Alps and Eddies. The complicated and often relictual distribution of this species in the far West seems to offer excellent opportunities for biogeographic interpretation, but its high vagility (i.e., potential colonizing ability) mandates caution.
Satyrium Californica
The California Hairstreak was not considered a riparian species until 1973, when the author found colonies in north Sacramento and eastern Yolo Counties, using valley oak (Quercus lobata ) as a host plant. No more such colonies have turned up, despite an assiduous search of oak groves (Walsh 1977). This may not mean no such colonies occur; Walsh's search was offseason, and the butterfly is easily missed—it spends much of its time in the trees, only coming down to feed around midday.
Although its existence was unsuspected before 1973, a valley oak ecotype of S . californica is hardly surprising. It commonly forms local ecotypes or ecological races, associated with various host plants of the genera Quercus and Ceanothus . At Rancho Cordova, Sacramento County, it is quite common—feeding on interior live oak (Quercus wislizenii ) and ignoring valley oak! In the subalpine zone on Packer's Peak in the Trinity Alps, it feeds on tobacco brush (Ceanothusvelutinus ). It is single-brooded everywhere, overwintering (and in the Valley, oversummering too!) as an egg.
Phenotypically, the valley oak ecotype is distinct from Coast Range foothill specimens, but not from the live oak-feeding Rancho Cordova population. It is not worthy of taxonomic recognition. So little valley oak vegetation remains that no one could be found to write a chapter on it for major synthesis of California vegetation studies.[5] Even if more than the three original colonies exist, the continuing deterioration of its habitat probably spells doom for this ecotype.
Lycaena xanthoides
The Great Copper presents the greatest mysteries in the Valley riparian fauna. Like Phyciodescampestris , it occurs in riparian broad-leaved vegetation, but also in moist grassland and at the edges of tule marsh. A glimpse at its northern California distribution (fig. 2) suggests that in the Valley it is restricted to the vicinity of the Sacramento River. There is no obvious reason for this. The butterfly is intensely colonial, and absent from a great many suitable-looking sites; yet it persists in the face of great disturbance. Its colonies occur on various soils, from sand to adobe clay; it is associated with at least six species of potential hosts (Rumex species, mostly introduced), none of which occurs in even a majority of its known colonies, except the very weedy R . crispus , whose range far exceeds the butterfly's.
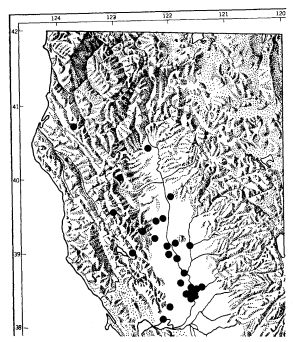
Figure 2.
Distribution of Lycaena xanthoides in and near the
Sacramento Valley, north of the Delta. Coastal and
Bay Area records, and the populations transitional
to L. editha from Dunsmuir to Weed, are omitted
(data in part from S.O. Mattoon; base map from
California Insect Survey).
Its distribution in the southern Valley has been carefully traced. It is common near Davis and Dixon (Solano County), and in the Yolo Bypass, West Sacramento, and Broderick (Yolo County). It crosses the Sacramento River at Discovery Park and ascends the American River to just beyond Highway 160, where it stops abruptly. As may be seen from the map, it has isolated (relict?) stations in interior Mendocino and Humboldt Counties, far from the Valley. It is common in the Delta and in vacant lots and marsh edges throughout the Bay Area, and extends to southern California. It is a montane species in the Tehachapis.
The Great Copper is completely unknown in the Sierra foothills and the adjacent east side of the Valley. In the montane and subalpine Sierra it is replaced by the smaller Lycaena editha , which extends northward at progressively lower elevations and appears to intergrade with L . xanthoides along Interstate 5 between Dunsmuir and Weed (Siskiyou County). L . editha is widespread in montane and subarid Ore-
[5] M.J. Barbour, Professor of Botany, University of California, Davis, personal communication.
gon, but true L . xanthoides occurs disjunctly in the Willamette Valley (Dornfeld 1980).
As with Phyciodescampestris , this species or species complex seems a good candidate for intensive biogeographic study. It has a single flight in late spring (May to early July). Univoltinism is characteristic of the entire subgenus, and winter is spent as an egg.
Prospects for the Future
Granted the continuing loss and fragmentation of riparian vegetation in the Sacramento Valley, the future of the butterfly fauna can be viewed in two lights. How likely is extinction at the level of populations or species? How are these extinction probabilities related to efforts to preserve riparian systems?
The generalized mesic character of the Valley riparian fauna argues against vulnerability on more than a local, or perhaps ultimately regional, scale. The fact that the fauna is largely derived from an Arcto-Tertiary biota adapted to mild climates with summer rain suggests that many of the species should still be common in such climates. In fact, we find them in the closest analogue available in California—the coastal fog belt. Polygoniasatyrus , for example, is very localized and rather rare in the Valley, but common and widespread in both Redwood and Douglas Fir associations coastwide. The Umber Skipper (Paratrytonemelane ) is an obligate riparian species in the Valley which can be found in every backyard in Berkeley.
Although several species like these might vanish from the Valley if riparian systems continue to disappear, they will leave large, healthy populations elsewhere. Many occur widely in the western half of North America, often with little geographic differentiation. Given the emphasis on endangered taxa (species and subspecies) in Federal conservation legislation, the Sacramento Valley riparian butterflies have little to offer conservationists looking for justifications for saving habitats. This is a pity, because butterflies have received a very favorable response in Washington. The sensitive entities are the colonial, endemic, or quasiendemic ones discussed in the preceding section. Two of these are not strictly riparian, and only one is a named subspecies.
Richard Arnold, in his Ph.D. dissertation,[6] studied the characteristics of most of the butterflies currently recognized as endangered or threatened. He found that low vagility, close association with a particular host plant and set of adult resources, and increasing fragmentation of the habitable range are attributes shared among them. However, most of the entities listed are Lycaenids, and a great many Lycaenids which are not presently endangered are local, sedentary, and monophagous. The critical variable is the amount (and contiguity) of breeding habitat. Many Lycaenids are potentially very vulnerable if their habitats become fragmented; other butterflies that resemble Lycaenids in population structure are also potentially in trouble. Let us see how these generalizations apply to the Valley riparian species.
Right now it seems unthinkable that Battus philenorhirsuta might become threatened; its annual population must run into the millions. Still, its reproductive capacity is low and it is completely dependent on a single host plant which is fairly habitat-specific. Notwithstanding these factors, a small colony (actually two colonies 2.4 km. (1.5 mi.) apart) has persisted on a handful of plants along the old channel of Putah Creek in Davis, which is now dry most of the year. These plants are growing in disturbed, mostly unshaded conditions and are subject to exceptional drought stress—yet they and their associated butterflies have remained essentially constant for the decade they have been under observation, even during the 1975–77 drought. In good years, surplus butterflies appear to emigrate from the colony. The Umber Skipper has disappeared from the same area since 1956. The persistence of this presumably relict population suggests that B . philenor can withstand much more fragmentation of habitat than it has yet had to face. However, as our most distinctive, spectacularly colorful, and taxonomically legitimate endemic it should be treated lovingly.
The other three species—Phyciodescampestris , Satyriumcalifornica , and Lycaena xanthoides —are colonial even without human interference. P . campestris fits all of Arnold's criteria for vulnerability except for its high vagility; individuals of both sexes are taken every year at Davis, 4.8 km. (3 mi.) from the nearest colony. They have indeed been taken at 1,525 m. (5000 ft.) on the Sierran west slope, within the range of P . c . montana and at least 80 km. (50 mi.) from the nearest known population in the Valley (Shapiro unpublished). The critical question here is how the spatial pattern of long-range dispersal maps onto the pattern of potentially colonizable sites.
Satyriumcalifornica and Lycaena xanthoides are quite sedentary, especially the former. Strays of xanthoides are recorded 16 km. (10 mi.) from breeding habitat (Shapiro unpublished). Colonies of xanthoides are numerous in the Davis-East Yolo-Sacramento area and gene flow remains likely. The status of the seemingly more isolated northern colonies is unknown. Its apparent rarity in the north Valley may merely reflect spotty collecting.
The long-term survival of the valley oak ecotype of S . californica is very unlikely. It perfectly matches Arnold's profile of an endangered butterfly. Had its phenotypic differentiation progressed to the same degree as the coastal dune endemics he studied, it would have been named and eligible for official Endangered
[6] Entomology, University of California, Berkeley, 1981.
status. But it did not. Even if additional colonies are located, the probabilities of inbreeding depression or random extinction are daunting. It requires oak reproduction for its own breeding. This imposes a limit on its tolerance of disturbance in the understory. It seems to be absent from those oak groves with the highest likelihood of preservation and selfrenewal. Its known sites are variously zoned for industrial development, and development as a waterfront regional park; and are located in a residential area where relict oaks have a low probability of surviving due to changes in drainage patterns.
The other side of the coin is the successful adaptation of a large part of the riparian butterfly fauna to the ersatz "riparian" systems in urban and suburban environments. Many of the most striking riparian butterflies, including Papilio rutulus , Nymphalis antiopa , Vanessaatalanta , and Atlideshalesus are breeding in street trees and gardens. I have observed 37 species of butterflies on my lot in one of the older subdivisions in Davis. None of the rarities breeds there, although I do get Phyciodescampestris in the garden regularly. So long as man provides mesic vegetation, the generalist multivoltine butterflies will use it.
The hope of finding relicts of the fauna of the pristine Valley has faded. We will probably never know what that fauna was like. Even so, there is plenty to be learned about the distribution and biology of the Valley riparian butterflies, and reason to hope that a few more Lepidopterists crossing the Valley enroute to somewhere else will be sufficiently intrigued to stop and take a look around.
Acknowledgments
I am indebted above all to Sterling O. Mattoon of Chico, who does collect in the Valley and who has graciously shared his data and perceptions with me. All of the data on figure 2 north of Knight's Landing and Marysville are his. I also thank Gary L. Rominger of the Sacramento County Department of Parks and Recreation for authorizing our ongoing studies of riparian butterflies along the American River Bikeway; everyone whose personal communications were cited in the text; and the Committee on Research and Department of Zoology, UCD, for funding work in the Sacramento Valley when they could have spent the money on research in Amazonia instead. Figure 1 was drawn by Ginny McDonald.
Literature Cited
Boisduval, J.B.A. de. 1869. Lépidoptères de la Californie. Annales de la Société Entomologique de Belgique 12:5–95.
Dornfeld, E. 1980. The butterflies of Oregon. 276 p. Timber Press, Forest Grove, Oregon.
Emmel, T.C., and J.F. Emmel. 1962. Ecological studies of Rhopalocera in a High Sierran community—Donner Pass, California. I: Butterfly associations and distributional factors. Journal of the Lepidopterists' Society 16(1):23–44.
Ford, E.B. 1975. Butterflies. Revised edition. 368 p. Collins-Fontana New Naturalist Series, London, England.
Larsen, T. 1976. The importance of migration to the butterfly faunas of Lebanon, East Jordan, and Egypt. Notulae Entomologicae 56:73–83.
Marshall, L.G. 1981. The Great American Interchange—an invasion-induced crisis for South American mammals. p. 133–230. In : M.H. Nitecki (ed.). Biotic crises in ecological and evolutionary time. 301 p. Academic Press, New York, N.Y.
Menendez, C.A. 1972. Paleofloras de la Patagonia. p. 129–184. In : M.J. Dimitri (ed.). La región de los Bosques AndinoPatagónicos. INTA, Buenos Aires, Argentina.
Opler, P.A., and R.L. Langston, 1968. A distributional analysis of the butterflies of Contra Costa County, California. Journal of the Lepidopterists' Society 22(2):89–107.
Ornduff, R. 1974. Introduction to California plant life. 152 p. University of California Press, Berkeley.
Petriella, B. 1972. Estudio de maderas petrificadas del Terciario Inferior del area de Chubut Central. Revista del Museo de La Plata, nueve serie, Paleontología 6:159–254.
Raven, P.H., and D.I. Axelrod. 1978. Origin and relationships of the California flora. 134 p. University of California Publications in Botany, vol. 72.
Sculley, R. 1973. The natural state. Davis New Review 1(1):3–13.
Shapiro, A.M. 1970. The butterflies of the Tinicum region. p. 95–104. In : Two studies of Tinicum Marsh. 124 p. Conservation Foundation, Washington, D.C.
Shapiro, A.M. 1974. The butterfly fauna of the Sacramento Valley, California. Journal of Research on the Lepidoptera 13:73–82, 115–122, 137–148.
Shapiro, A.M. 1975. Supplementary records of butterflies in the Sacramento Valley and Suisun Marsh, lowland central California. Journal of Research on the Lepidoptera 14: 100–102.
Shapiro, A.M. 1977. Photoperiod and temperature in phenotype determination of Pacific Slope Pierini: biosystematic implications. Journal of Research on the Lepidoptera 16:193–200.
Sharpiro, A.M., and A.R. Shapiro. 1973. The ecological associations of the butterflies of Staten Island (Richmond County, New York). Journal of Research on the Lepidoptera 12:65–128.
Shapiro, A.M., C.A. Palm, and K.L. Wcislo. 1981. The ecology and biogeography of the butterflies of the Trinity Alps and Mount Eddy, northern California. Journal of Research on the Lepidoptera 18:69–152.
Thompson, K. 1961. Riparian forests of the Sacramento Valley, California. Annals of the Association of American Geographers 51:294–314.
Tilden, J.W. 1959. The butterfly associations of Tioga Pass. Wasmann Journal of Biology 17:249–271.
Volkheimer, W. 1971. Aspectos paleoclimatológicos del Terciario Argentino. Revista del Museo de Ciencias Naturales "Bernardino Rivadavia," Buenos Aires, serie Paleontológica 1:243–262.
Walsh, J.B. 1977. Report on the distribution and status of Quercuslobata groves in California which may support an ecotype of Satyrium californica . Atala 5:7–12.
The Status of Ecological Research on the Mammal Fauna of California's Central Valley Riparian Communities[1]
Gene R. Trapp, Gail L. Linck, and Edward D. Whisler[2]
Abstract.—An extensive literature search was conducted, and various local authorities questioned, regarding: 1) the species of mammals present in the riparian communities of California's Central Valley; and 2) the status of information on these species in relation to the communities. The result was a realization that the mammal fauna of these riparian communities has been studied very little. A checklist of 55 mammalian species was developed. It appears that about half the species listed have had no ecologically oriented research, and most others have had little.
Introduction
Each fall semester from 1970 to 1980, the senior author examined the results of comprehensive bibliographic searches on the biology of most species of California mammals. These searches were conducted under his supervision by mammalogy students at California State University, Sacramento (Trapp 1971–80). Much less ecological research has been published on the approximately 173 non-cetacean California species perse than might be expected, considering the relative abundance of research institutions in the state. Less ecologically oriented research had been published on mammals of Central Valley riparian communities than on those of the rest of the state. Riparian communities are rapidly being lost (Gaines 1976; Sands 1977; Warner 1979); those interested in their preservation would be helped by a clarification of the status of our knowledge of the mammal fauna of these communities. Our objectives in this study were: 1) to revise the checklist of mammalian species begun by Roberts etal . (1977) to include all riparian communities of the entire Central Valley; and 2) to determine the status of ecologically oriented research on these species.
Procedure
The expression "riparian communities" is used here according to Warner (1979). We considered these communities to fall within the boundaries of the Central Valley "California Prairie" according to Küchler (1977).
We used the following sources to construct the checklist: Roberts etal . (1977); Grinnell etal . (1937); Ingles (1965); Williams (1979, 1981); Hall (1981); Elems and Medeiros (1981); and various other reports, e.g., Stone (1976), Brumley (1976), Schempf and White (1977). In addition to drawing upon the senior author's personal experience, we questioned the following authorities about their knowledge of mammals present in the Central Valley or their local areas: Robert Rudd;[3] Robert Schwab, Rex Marsh, Ron Cole;[4] Stan Elems;[5] Howard Leach and William Grenfell;[6] Terry Mansfield;[7] Gary Shook;[8] and Daniel Williams.[9]
[1] Paper presented at the California Riparian Systems Conference. [University of California, Davis, September 17–19, 1981].
[2] Gene R. Trapp is Associate Professor, Gail L. Linck is a Graduate Student, and Edward D. Whisler is a Senior in the Department of Biological Sciences, California State University, Sacramento.
[3] Robert Rudd. 1981. Personal conversation. Department of Zoology, University of California, Davis.
[4] Robert Schwab, Rex Marsh, and Ron Cole. 1981. Personal conversations. Division of Wildlife and Fisheries Biology, University of California, Davis.
[5] Stan Elems. 1981. Personal conversation. Department of Biology, Modesto Junior College, and Great Valley Museum, Modesto, Calif.
[6] Howard Leach (retired) and William Grenfell. 1981. Personal conversations. Wildlife Investigations Laboratory, California Department of Fish and Game, Sacramento.
[7] Terry Mansfield. 1981. Personal conversation. Big Game Investigations, California Department of Fish and Game, Sacramento.
[8] Gary Shook. 1981. Personal conversation. Woodson Bridge State Recreation Area, Corning, Calif.
[9] Daniel Williams. 1981. Personal communication. Department of Zoology, California State University, Stanislaus, Turlock.
We searched the literature for species on the checklist using two approaches. The first and less productive was a "habitat approach", using key words to search the following sources for the dates indicated:
Zoological Record: 1940–1975 (latest issue) for river, fresh water habitat, riparian habitat, terrestrial habitat, and (in older issues) ecology and habitats;
Wildlife Abstracts: 1935-June 1981 for natural areas and refuges, faunas, communities, and wetlands and wildlife. Prior to 1970—mammals in general, biotas, faunas, and populations;
Wildlife Reviews: as per Wildlife Abstracts;
Biological Abstracts: 1974-early 1981 for riparian, riverine.
The second and more productive approach was a search for articles about the mammal species on the checklist. We went through the California State University Bibliographies of Selected Mammalian Species (Trapp 1971–80), which generally reach back into the 1930–40 period, and updated each to the dates indicated below with the following sources:
Zoological Record: through 1975;
Wildlife Abstracts: 1935–June 1981;
Wildlife Review: 1935–June 1981;
Mammalian Species: Numbers 1–156 (May 1981);
Journal of Mammalogy indices: through 1980.
Some specialized searches made by others were examined also: Newberry (1973), Trapp and Hallberg (1975), McGrade (1978), and Antonius (1981).
Results and Discussion
The checklist (table 1) contains 55 species compared to 39 in the initial list begun by Roberts etal . (1977). The amount of evidence for the following species' presence in the natural riparian communities of the Central Valley varies. However, Ingles (1965) shows the following species absent or less widely distributed than reported by the authority indicated in parentheses:
broad-handed mole (Elems 19815 );
western (greater) mastiff bat (Hall 1981; Elems 19815 );
western gray squirrel (Stienecker 1977);
porcupine (Rudd 1981,3 Shook 19818 );
ringtail (Naylor and Wilson 1956; Michny etal . 1975; Stone 1976; Brumley 1976; Belluomini and Trapp, in press);
mountain lion (Brumley 1976; Elems 19815 );
black-tailed and California mule deer (Dasmann 1968; Brumley 1976; Mansfield 19817 ); and
feral hog (Dasmann 1968; Mansfield 19817 ).
The presence of these species needs to be better documented for the riparian communities of the Central Valley. This is also true for the following species:
|
brush rabbit
eastern gray squirrel
fox squirrel
San Joaquin pocket mouse
Heermann's kangaroo rat
deer mouse
dusky-footed woodrat
spotted skunk.
The fox squirrel seems to be spreading out into the suburbs from what in the past has been an urban habitat, e.g., in Sacramento suburbs. On 2 July 1981 Michael Lacy and Trapp saw one on the west side of the Sacramento River on the outskirts of Knights Landing (Yolo County).
The nutria (Myocastorcoypus ), though released in the past at various points in California (Ingles 1965), is thought to have been exterminated by the California Department of Agriculture (Leach 1981;[6] Marsh 1981[4] ). However, Williams (1981,[9] ) has had reports from ranchers and biologists that nutria are still present in the San Joaquin Valley.
The spread of the black rat, Norway rat, house mouse, and feral hog into natural riparian communities is not well documented. The feral hog occurs along the upper Sacramento River south of Red Bluff in Tehama County; on the Colusa National Wildlife Refuge in Colusa County; and on Grizzly Island in Suisun Marsh, Solano County (Dasmann 1968; Mansfield 1981[7] ); it is apparently extending its range.
The tule elk presently exists free-living only on Grizzly Island at the northeast end of Suisun Bay, Solano County. The only other potentially riparian herd of tule elk is in a 308-ha. (761-ac.) enclosure of annual grassland, wooded sloughs, and marsh on the San Luis National Wildlife Refuge along the San Joaquin River in Merced County (Dasmann 1968; USDI Bureau of Land Management 1979; Leach and Grenfell 1981[6] ).
The black-tailed deer is found along the Sacramento River and its tributaries, extending south to the Cosumnes River/Stockton region. There its range overlaps and the deer hybridizes with the California mule deer, which occurs along drainages in the San Joaquin system (Mansfield 1981[7] ). Dasmann (1968) fails to show these deer in the San Joaquin Valley or in the lower Sacramento Valley.
The following species are not included on the checklist, although they may occur in Central Valley riparian communities. Hall (1981) extrapolates their ranges into the Central Valley, but Ingles (1965) does not. These species are:
small-footed myotis (Myotissubulatus )
little brown myotis (M . lucifugus )
long-legged myotis (M . volans )
fringed myotis (M . thysanodes )
long-eared myotis (M . evotis )
The Status of Research
Authoritative contributions to the knowledge of mammalian ecology in the riparian communities of the Central Valley are relatively few, considering the number of species and the number of research institutions in the state. "Furbearing Mammals of California" (Grinnell etal . 1937) still stands as the most important contribution regarding furbearers of the state, though it needs to be updated. The information is largely of a natural history nature with many contributions made by interviewed trappers.
"Mammals of the Pacific States" (Ingles 1965) is another major contribution to mammalogy in California. It contains general information on mammals, a key to the species, distribution maps, and information from the literature and the author's experience summarizing the biology and natural history of the species. This work, however, makes a limited contribution to the riparian mammal ecology in the Central Valley and is in need of revision. Two other helpful works of synthesis, including information collected by game department biologists, are California Department of Fish and Game (DFG) booklets "Big Game of California" (Dasmann 1968), in need of revision, and "Furbearers of California" (Seymour 1977).
Limited information on the distribution of mammals in riparian communities has been included in unpublished reports by the DFG (e.g., Leach 1963, Michny etal . 1975, Stone 1976, and Brumley 1976). Brumley's report is especially useful because it includes a list of 41 mammal species observed in the Upper Butte Basin northeast of Colusa. Schempf and White's (1977) survey of six furbearers in the mountains of northern California makes some brief but useful comments pertaining to the Central Valley on the distribution of ringtail, river otter, and red fox.
Turning to the scientific journals for research papers on Central Valley riparian mammals, we found no information for about half the species on the checklist and very limited amounts for the rest. We found research reports ecologically oriented to riparian communities only for the species mentioned below.
Opossum (Introduced)
Campbell (1981a) reported capturing some albinotic individuals in north San Joaquin County near the Mokelumne River. He also studied activity patterns of captive young Central Valley opossums (kept in outdoor cages in north Sacramento) correlated with elements of weather and photoperiod (Campbell 1981b). Reynolds (1952) reported on reproduction in Central Valley opossums.
Black-tailed Hare
Hardy etal . (1977) reported on natural and experimental arboviral infections in this species along the Sacramento River in Butte County.
California Ground Squirrel
Owings and his associates at the University of California, Davis, have made several contributions to the literature on this species. Owings and Borchert (1975) studied correlates of burrow location; Owings etal . (1977) described general behavior; Coss and Owings (1978) reported on snake-directed behavior by snake-naive and experienced ground squirrels; and Owings etal . (1979) described time budgets of this species during reproduction.
Eastern Gray Squirrel and Fox Squirrel (Introduced)
Byrne (1979) studied distribution and ecology of eastern gray squirrel and fox squirrel in northern California.
Western Gray Squirrel
Ingles (1947) studied several aspects of the life history of western gray squirrel, including food habits, in a Sacramento Valley riparian forest on Big Chico Creek at Bidwell Park in Chico, Butte County. Stienecker (1977) provided an improved range map (cf. Ingles 1965) in his paper on food habits, but had no study sites in the Central Valley. Swift (1977) studied the reproductive cycle of this species in Butte County.
Beaver
For this species, there are only status reports by Tappe (1942); Cram (1951); and Lee (1977).
Deer Mouse
Fuller (1978) mentioned Peromyscusmaniculatus as part of the diet of a gray fox at Davis, Yolo County. Biggerstaff (1977) studied swimming behavior of this species and three other mice (Reithrodontomysmegalotis , Microtus californicus , and Musmusculus ) found in the Sacramento/San Joaquin River delta.
California Vole
Haynie (1974) studied high population density stress and resistance to pasteurellasis in the California vole in outdoor enclosures near Sacramento. Smith (1975) studied the association between small mammal populations and certain plant communities in the Graylodge Wildlife Area of Butte County.
Muskrat (Introduced)
Distribution and status of muskrat were reported on by Twinning and Hensley (1943), Seymour (1954), and Lee (1977). Messa (1981) studied population dynamics and home range of this species along irrigation canals in east Yolo County, northeast of Davis.
Norway Rat and Black Rat (Introduced)
Brooks and Barnes (1972) reported on an outbreak and decline of Norway rat populations in California rice fields. Stroud (1982) studied population dynamics of Norway and black rats in a riparian habitat on Putah Creek in Yolo County. The role of rodents in plague ecology was examined by Nelson (1980).
Coyote
Dow (1975) analyzed four coyote populations in several counties of the Sacramento Valley region. Crellin (1977) related helminths in coyotes to ecological factors in the San Joaquin Valley.
Red Fox (Introduced)
Gray (1977) and Gould (1980) reported on distribution and status of red fox; Roest (1977) examined taxonomic status compared to the native Sierran red fox.
Gray Fox
Lee (1977) reported on status, and Fuller (1978) described variable home-range sizes in four females of this species in relation to habitat on Putah Creek at Davis, Yolo County. Hallberg and Trapp (1981) described temporal behavior and home-range characteristics in two males and two females east of Davis on Putah Creek.
Ringtail
Belluomini and Trapp (1981) summarized earlier ringtail sightings, plus their own work, and reported on distribution and density of this species in the Central Valley, which Grinnell etal . (1937), Ingles (1965), and Hall (1981) indicated was not present to the extent that it is.
Raccoon, Mink, and Badger
Lee (1977) reported on these species' status as furbearers.
Spotted Skunk
Mead (1962) studied several aspects of this species' life history. Orloff (1980) reported on present distribution.
Striped Skunk
Mead (1962) also studied several aspects of striped skunk natural history. Gray (1975) studied home range, movements, activity, and den characteristics; while Peck (1980) studied the family unit's activity patterns, home range, and denning habits, both along Putah Creek, Yolo County. Belluomini (1980) reported on the status of this species.
River Otter
Gould (1977) reported on river otter status; and Grenfell (1978) studied their food habits in Suisun Marsh.
Feral House Cat (Introduced)
Hubbs (1951) reported on food habits of the feral house cat in the Sacramento Valley.
Feral Hog (Introduced)
Dasmann (1968) commented on status and distribution of feral hog; and Barrett (1978) studied several aspects of ecology, including movements and food habits, on the Dye Creek Ranch southeast of Red Bluff in Tehama County.
Tule Elk
Dasmann (1968) commented on this species' status and distribution. A more recent report was made by the USDI Bureau of Land Management (1979).
Summary
Roberts etal . (1977) offered their mammal species checklist for the Sacramento Valley riparian forests as a starting point. The checklist presented here has been refined and broadened to include the San Joaquin Valley. It also is offered to interested biologists for further refinement. We have found that the composition of the mammal fauna of the Central Valley's riparian communities is poorly documented.
More research is also needed to improve our knowledge of life histories of riparian mammals, and the ecological relationships between them and the communities they inhabit in the Central Valley. Opportunities for conducting this research have been diminishing with the destruction of natural riparian communities, so we recommend that field biologists turn their attention without delay to the remaining opportunities for research in this poorly understood area.
Acknowledgments
We would like to thank Daniel F. Williams, Stan W. Elems, Carolyn Stallard, and Jo Ellen Diem for reading the manuscript and offering helpful suggestions. We also thank Richard Warner for suggesting this project and guiding in its preparation, and Gordon Gould for providing information.
Literature Cited
Antonius, D. 1981. Master's thesis bibliography of nongame animals. Nongame Wildlife Investigations Job Final Report, Project No. W-54-R-12, Job III-6. 92 p. California Department of Fish and Game, Sacramento.
Barrett, R.H. 1978. The feral hog on the Dye Creek Ranch, California. Hilgardia 46(9): 283–355.
Belluomini, L. 1980. Status of the striped skunk in California. Nongame Wildlife Investigations Progress Report, Project No. W-54-R-12, Job I-8. 6 p. California Department of Fish and Game, Sacramento.
Belluomini, L., and G.R. Trapp. 1981. Ringtail distribution and abundance in the Central Valley of California. In : R.E. Warner and K.M. Hendrix (ed.). Proceedings of the California Riparian Systems Conference. [University of California, Davis, September 17–19, 1981]. University of California Press, Berkeley.
Biggerstaff, C.E. 1977. A comparison of swimming behavior in four species of mice found in the Sacramento-San Joaquin River delta of California. Unpublished M.S. Thesis. University of the Pacific, Stockton, Calif. 35 p.
Brooks, J.E., and A.M. Barnes. 1972. An outbreak and decline of Norway rat populations in California rice fields. Calif. Vector Views 19(2):5–14.
Brumley, T.D. 1976. Upper Butte Basin study: 1974–75. Admin. Report No. 76–1. 30+ p. California Department of Fish and Game, Wildlife Management Branch, Sacramento.
Byrne, S. 1979. The distribution and ecology of the non-native tree squirrels Sciurus carolinensis and Sciurusniger in northern California. Ph.D. Dissertation. University of California, Berkeley. 196 p.
Campbell, M.R. 1981a. Records of albinotic opossum from central California. Southwest Nat. 25(4):560.
Campbell, M.R. 1981b. Activity patterns of captive opossum young (Didelphisvirginiana ) correlated with elements of weather and photoperiod. Unpublished M.S. Thesis, California State University, Sacramento. 134 p.
Coss, R., and D.H. Owings. 1978. Snake-directed behavior by snake naive and experienced California ground squirrels in a simulated burrow. Zeit. Tierpsychol. 48(4):421–435.
Cram, D.D. 1951. The status of beavers in the delta area of the San Joaquin River, San Joaquin County, California. Unpublished M.A. Thesis, University of the Pacific, Stockton, Calif. 77 p.
Crellin, J. 1977. Helminths of the coyote in the San Joaquin Valley, California, with some ecological and epidemiological considerations. Unpublished M.A. Thesis. California State University, Fresno. 48 p.
Dasmann, W.P. 1968. Big game of California. 56 p. California Department of Fish and Game, Sacramento.
Dow, R.J. 1975. Analysis of four populations of free ranging coyotes in California. Unpublished M.S. Thesis, University of California, Davis. 54 p.
Elems, S.W. and J. Medeiros. 1981. Flora and fauna of Caswell State Park, Ripon, California. Regional Biota Series No. 1, Great Valley Museum, Modesto, Calif. 20 p.
Fuller, T.K. 1978. Variable home-range sizes of female gray foxes. J. Mamm. 59(2):446–449.
Gaines, D.A. 1976. Abstracts from the conference on the riparian forests of the Sacramento Valley. 25 p. Davis and Altacal Audubon Societies, P.O. Box 886, Davis, Calif.
Gould, G. 1977. Status of the river otter in California. Nongame Wildlife Investigations Status Report. 4 p. California Department of Fish and Game, Sacarmento.
Gould, G. 1980. Status of the red fox in California. Nongame Wildlife Investigations Progress Report, Project No. W-54-R-12, Job I-8. 3 p. California Department of Fish and Game, Sacramento.
Gray, R.L. 1975. Home range, movements, activity, and den ecology of the striped skunk (Mephitis mephitis ) in the Sacramento Valley, California. Unpublished M.S. Thesis, California State University, Sacramento. 36 p.
Gray, R.L. 1977. Extensions of red fox distribution in California. Calif. Fish and Game 63(1):58.
Grenfell, W.E., Jr. 1978. Food habits of the river otter in Suisun Marsh, Central California. P. 65–73. In : Proceedings of the Cal-Neva Wildlife Conference. [February 2–4, 1978]. California Department of Fish and Game, Rancho Cordova.
Grinnell, J., J. Dixon, and J.M. Linsdale. 1937. Fur-bearing mammals of California. 2 volumes. 777 p. University of California Press, Berkeley.
Hall, E.R. 1981. The mammals of North America (second edition). 2 volumes. 1181 p. John Wiley and Sons, Inc., New York, N.Y.
Hallberg, D.L., and G.R. Trapp. 1981. Gray fox (Urocyoncinereoargenteus ) temporal and spatial activity in a riparian-agricultural habitat in California's Central Valley. In : R.E. Warner and K.M. Hendrix (ed.). Proceedings of the California Riparian Systems Conference. [University of California, Davis, September 17–19, 1981]. University of California Press, Berkeley.
Hardy, J.L., M. Milby, M.E. Wright, A.J. Beck, S.B. Presser, and J.P. Bruen. 1977. Natural and experimental arboviral infections in a population of black-tail jackrabbits along the Sacramento River, Butte County, California (1971–74). J. Wildlife Disease 13(4): 383–392.
Haynie, T. 1974. High population density stress and resistance to pasteurellasis in the California vole, Microtuscalifornicus . Unpublished M.A. Thesis, California State University, Sacramento. 53 p.
Hubbs, E.L. 1951. Food habits of feral house cats in the Sacramento Valley. Calif. Fish and Game 37:177–189.
Ingles, L.G. 1947. Ecology and life history of the California gray squirrel. Calif. Fish and Game 33(3):139–158.
Ingles, L.G. 1965. Mammals of the Pacific States. 506 p. Stanford University Press, Stanford, Calif.
Küchler, W.A. 1977. Map of the natural vegetation of California. p. 909. In : M.G. Barbour and J. Major (ed.). Terrestrial vegetation of California. 1002 p. John Wiley and Sons, Inc., New York, N.Y.
Leach, H.R. 1963. A reconnaissance report on the lower Feather River, with special reference to the effects of water developments on fish, wildlife, and recreation. Report LFR-1063. 97+ p. California Department of Fish and Game, Water Projects Branch, Sacramento.
Lee, R.C. 1977. Status of harvested furbearers in California—badger, beaver, gray fox, mink, muskrat, and raccoon. Nongame Wildlife Investigations Progress Report, Project No. W-54-R-9, Job II-1.0. 29 p. California Department of Fish and Game, Sacramento.
McGrade, H. 1978. Research bibliography: Gray fox, spotted skunk, and striped skunk. Unpublished report, Department of Biological Sciences, California State University, Sacramento. 23 p.
Mead, R.A. 1962. Some aspects of the life histories of the spotted and striped skunks. Unpublished M.A. Thesis, University of California, Davis. 61 p.
Messa, D.J. 1981. Population dynamics of Sacramento Valley muskrats. Unpublished M.S. Thesis, California State University, Sacramento. 66 p.
Michny, F.J., D. Boos, and F. Wernette. 1975. Riparian habitats and avian densities along the Sacramento River. Admin. Report No. 75-1. 42 p. California Department of Fish and Game, Sacramento.
Naylor, A.E., and G.W. Wilson. 1956. Unusual occurrence of the ring-tailed cat. Calif. Fish and Game 42(3):231.
Nelson, B.C. 1980. Plague studies in California—the roles of various species of sylvatic rodents in plague ecology in California. Proc. Vertebr. Pest Conference 9:89–96.
Newberry, D.W. 1973. A contribution toward a bibliography on California furbearers. Special Wildlife Investigations Progress Report, Project W-54-R-5, Job II-5.7. 10 p. California Department of Fish and Game, Sacramento.
Orloff, S. 1980. Spotted skunk distribution study. Nongame Wildlife Investigations Job Final Report, Project No. W-54-R-12, Job IV-1. 13 p. California Department of Fish and Game, Sacramento.
Owings, D.H., and M. Borchert. 1975. Correlates of burrow location in Beechey ground squirrels. Great Basin Nat. 35(4):402–404.
Owings, D.H., M. Borchert, and R. Virginia. 1977. The behavior of California ground squirrels. Animal Behavior 25(1):221–230.
Owings, D.H., R. Virginia, and D. Paussa. 1979. Time budgets of California ground squirrels during reproduction. Southwest. Nat. 24(1):191–195.
Peck, B. 1980. Activity patterns, home range, denning habits, and observations of the family unit of the striped skunk (Mephitismephitis ) in the Sacramento Valley, California. Unpublished M.S. Thesis, University of California, Davis. 86 p.
Reynolds, H.C. 1952. Studies on reproduction in the opossum (Didelphisvirginiana ). Univ. Calif. Publ. Zool. 52:223–284.
Roberts, W.G., J.G. Howe, and J. Major. 1977. A survey of riparian forest flora and fauna in California. p. 3–19. In : A. Sands (ed.). Riparian forests in California: their ecology and conservation. Institute of Ecology Pub. 15, University of California, Davis. 122 p.
Roest, A.I. 1977. Taxonomic status of the red fox in California. Nongame Wildlife Investigations Job Final Report, Project No. W-54-R-9, Job II-1.3. 15 p. California Department of Fish and Game, Sacramento.
Sands, A. (ed.). 1977. Riparian forests in California: their ecology and conservation. Institute of Ecology Pub. 15, University of California, Davis. 122 p.
Schempf, P.F., and M. White. 1977. Status of six furbearer populations in the mountains of northern California. 51 p. US Department of Agriculture Publications, Forest Service, California Region.
Seymour, G. 1954. Recent extension of the range of muskrats in California. Calif. Fish and Game 40(4):375–384.
Seymour, G. 1977. Furbearers of California. 53 p. California Department of Fish and Game, Sacramento.
Smith, M.F. 1975. Small mammal populations associated with certain plant communities in Graylodge Wildlife Area. Unpublished M.A. Thesis. California State University, Chico. 39 p.
Stienecker, W.E. 1977. Supplemental data on the food habits of the western gray squirrel. Calif. Fish and Game 63(1):11–21.
Stone, T.B. 1976. Observations on furbearers within the riparian habitat of the upper Sacramento River. Memorandum Report. 12 p. California Department of Fish and Game, Sacramento.
Stroud, D. 1982. Population dynamics of Rattusrattus and R . norvegicus in a riparian habitat. J. Mamm. 63(1):151–154.
Swift, R.J. 1977. The reproductive cycle of the western gray squirrel in Butte County, California. Unpublished M.A. Thesis, California State University, Chico. 78 p.
Tappe, D.T. 1942. The status of beavers in California. Game Bulletin No. 3., California Division of Fish and Game. 59 p.
Trapp, G.R. (ed.). 1971–80. Bibliographies of selected mammalian species. Library, California State University, Sacramento.
Trapp, G.R., and D.L. Hallberg. 1975. Ecology of the gray fox (Urocyoncinereoargenteus ): a review. p. 164–178. In : M.W. Fox (ed.). Wild canids. 508 p. VanNostrand Reinhold Co., New York, N.Y.
Twinning, H., and A.L. Hensley. 1943. The distribution of muskrats in California. Calif. Fish and Game 29(2):64–78.
USDI Bureau of Land Management. 1979. Third annual report to Congress: the tule elk. 32 p. California State Director, Bureau of Land Management, Sacramento.
Warner, R.E. 1979. The California riparian study program. Phase I: Background studies and program design for Phase II. 177 p. California Department of Fish and Game, Planning Branch, Sacramento.
Williams, D.F. 1979. Checklist of California mammals. Annals of Carnegie Museum 48(23): 425–433.
Williams, D.F. In press. Mammalian species of special concern in California. Nongame Wildlife Investigations Job Final Report, Project No. E-W-4, Job IV-14.1. California Department of Fish and Game, Sacramento.
Sensitive, Threatened, and Endangered Mammals of Riparian and other Wetland Communities in California[1]
Daniel F. Williams and Kerry S. Kilburn[2]
Abstract.—Studies of the distribution, habitat requirements, and population status of species and subspecies of mammals in California were conducted in order to identify taxa threatened with extinction. Investigations were limited to taxa without current state or federal Rare, Threatened, or Endangered status. Twenty-one species and subspecies of mammals confined to or dependent upon riparian and other wetland communities were identifed as being especially vulnerable to loss of habitat and facing potential threats of extinction. These taxa are grouped into four categories depending upon the apparent nature and proximity of the threats to their populations. Destruction of riparian and other wetland communities is the principal factor jeopardizing all 21 taxa. Preservation of these and other members of riparian and other wetland communities can probably be accomplished most efficiently by an integrated approach that focuses on preserving biotic communities rather than single species. Herein we outline the elements of such a plan.
Introduction
Of the 502 recent native species and subspecies of land mammals in California (Hall 1981), approximately 25% (133 taxa) are limited to or largely dependent upon riparian and other wetland communities. No other general type of mammalian habitat in California approaches riparian and other wetland communities in importance to mammals, and none has been so diminished in extent and degraded in quality (Warner 1979). As a result, populations of mammalian species dependent upon freshwater and tidal riparian wetland communities have declined markedly in size in nearly every region within California. Whitetailed deer (Odocoileus virginianaochroura and O . v . couesi ) have become extinct in California in this century (Williams in press). Populations of one tideland species, salt marsh harvest mice (Reithrodontomysraviventris raviventris and R . r . halicoetes ) and one freshwater wetland species, the Amargosa vole (Microtuscalifornicusscirpensis ), are listed as Endangered (California Department of Fish and Game 1980); several other riparian and wetland species are seriously jeopardized by destruction and degradation of their habitats.
Concern over the rapid loss of biotic communities within California and the resulting threats to wildlife prompted the Nongame Wildlife Investigations Unit of the California Department of Fish and Game (DFG) to commission a study of potentially threatened populations of mammals within the state. Williams initiated that study in 1979 and filed the final report to DFG in September 1981 (Williams in press). The investigation was limited to native species of land mammals without state or federal Rare, Threatened, or Endangered status. Populations of 52 species and subspecies were identified as being potentially threatened with extinction; 21 of these are limited to or principally dependent upon riparian and wetland communities.
This report summarizes the investigations into the current population status of those mammalian species confined to or dependent upon riparian and other wetland communities in California.
Methods
Taxa believed to be extinct and species concurrently being investigated by the DFG were excluded from the investigation. To be included on the final list of concern, the entire California population of a taxon had to be potentially
[1] Paper presented at the California Riparian Systems Conference. [University of California, Davis, September 17–19, 1981].
[2] Daniel F. Williams is Professor of Zoology and Kerry S. Kilburn is a student in the Department of Biological Science; both are at California State College, Stanislaus, Turlock.
jeopardized with extinction, given continuation of current trends in diminishment and degradation of habitat, harvest or persecution, or other factors threatening populations. Thus, locally depleted populations of more wide-ranging taxa were excluded, with a single exception: an isolated population of the Sierra Nevada mountain beaver (Aplodontiarufa californica ), living in tufa deposits along Lee Vining Creek near where it historically entered Mono Lake (Steele in press), was included because of its unique habitat.
Initially, a working list of 86 candidate species and subspecies was assembled. Information on life history, habitat requirements, past and present distributions, systematic status, probable population status, and the nature of potential threats was gathered for each candidate, from the literature and other sources (see below). Letters and questionnaires requesting information on potentially threatened mammals were sent to all members of the American Society of Mammalogists residing in California, and to other, selected persons in state and federal agencies, universities, and natural history museums. A number of persons were contacted by telephone for inquiries about selected taxa and developments affecting wildlife habitats in certain areas of the state. Williams visited and obtained distribution data from 17 natural history museums which collectively contain the great majority of mammal specimens from California. Distribution data on some taxa were also obtained from 18 other mammal research collections (see Williams in press).
Most areas in the state where loss of habitat was thought to pose a threat to one or more candidate species were visited. Some preliminary field work to determine presence and abundance of candidate species was conducted, but time and funding did not permit detailed or extensive field work. Rather, the objective of the investigations was to develop a list of species, in priority categories, which would be used to determine the disbursement of limited funds for detailed investigations in the field and for other administrative decisions.
Common and scientific names used in this report are from Williams (1979). Vernacular names for subspecies are included because subspecies can be accorded state and federal Rare, Threatened, or Endangered status. These names are from Grinnell (1933), or those coined by the authors of more recently described species and subspecies, or, when no name was available, by Williams (in press). See Williams (ibid .) for detailed remarks on the taxonomy used here.
Refer also to Williams (ibid .) for a detailed account of methods, remarks on systematic status, recommendations for management actions, and documentation of distribution records.
Results
Twenty-one species and subspecies of mammals of riparian and other wetland communities were found to face potential threats of extinction. The major factor jeopardizing each of these populations is loss and degradation of habitat. Each taxon is assigned to one of four categories according to the apparent proximity of the threats to remaining populations (table 1). The categories are described below.
Category l.—Species are considered to be potentially endangered as defined by the federal Endangered Species Act of 1973.[3] Immediate action to stop loss and degradation of habitat for these species is needed. Field investigations to establish status and baseline population data should be carried out as rapidly as possible.
Category 2.—Species may be threatened or endangered as defined in the federal Endangered Species Act, but the threats of extinction seem less imminent than for species in category 1. Priority in management actions should be given to halting loss and degradation of habitat and establishing baseline data on populations.
Category 3.—Species probably do not warrant Endangered status now and appear not to be under proximate threats of extinction. If current trends in loss and degradation of habitat continue, however, they could quickly become endangered. These species may merit Rare (state) or Threatened (federal) status under current regulations. The principal administrative actions required are to initiate field investigations into population status and to consider the habitat needs of these species in land development and resource management plans.
Category 4.—Species are considered to be sensitive or vulnerable to disturbances, including loss and degradation of habitat, overharvesting, and other factors. Principal administrative actions needed include special considerations for these species in land development and resource management decisions, and protection from overharvest.
Distribution
Table 1 also briefly lists the distribution of each taxon. Note that five species found principally or wholly along the Colorado River in California are considered to be jeopardized. Of the five, only the Yuma mountain lion (Felisconcolor browni ) probably ranges far beyond the immediate vicinity of the river valley (ibid .), although it appears to be dependent upon the riparian community. Two of the species are restricted to the tidal marshes in the coastal region of the Los Angeles Basin, and two others are confined to the salt marsh communities in the San Francisco Bay area. Four of the poten-
[3] P.L. 93–205.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
tially jeopardized species are found in the wetland and riparian communities of the San Joaquin Valley. Of these four, only the golden beaver (Castorcanadensissubauratus ) ranges beyond the San Joaquin Valley. The golden beaver is the only species listed here which may also face a serious threat from overharvesting.
These four areas, the Colorado River Valley, the San Joaquin Valley, and the tidal marshes of the Los Angeles Basin and San Francisco Bay, are viewed as special problem areas in terms of loss and degradation of riparian and wetland communities. Degradation of riparian and other wetland communities has, however, diminished mammalian habitats throughout all areas of California (table 1).
Jeopardized Species
The habitats of the 21 taxa listed in table 1 are briefly outlined below. In many cases, little or no data were available for the taxa of concern.
Salt Marsh Wandering Shrew
The salt marsh wandering shrew (Sorexvagrans halicoetes ) occupies the medium-high marsh about 1.8–2.4 m. (6–8 ft.) above sea level and lower marsh areas not regularly inundated, characterized by abundant driftwood and other debris scattered among Salicornia . It requires dense cover, abundant food (invertebrates), suitable nesting sites, and fairly continuous ground moisture (Johnston and Rudd 1957).
San Bernardino Dusky Shrew
The San Bernardino dusky shrew (Sorexmonticolusparvidens ) is probably similar in its habitat association to the populations in the Sierra Nevada; no information on its specific requirements, however, is available. In the Sierra Nevada, dusky shrews are associated with riparian and wetland communities from the upper mixed conifer zone to the timberline (Williams in press).
Buena Vista Lake Shrew
The Buena Vista Lake shrew (Sorexornatus relictus ) occupied marshes on the perimeter of the historic Lake Buena Vista (Grinnell 1933). It may occupy dense vegetation along streams and sloughs and around the perimeter of tule marshes in the Tulare Basin, although nothing has been recorded about its habitat (Williams in press).
Southern California Salt Marsh Shrew
The Southern California salt marsh shrew (Sorex ornatussalicornicus ) occurs in coastal marshes and probably requires fairly dense groundcover, nesting sites above mean high tide and free from inundation, and fairly moist surroundings. Nothing has been recorded about its habitat requirements (ibid .).
Suisun Shrew
The Suisun shrew (Sorexornatussinuosus ) appears to require dense, low-lying cover where invertebrates are abundant. It typically inhabits tidal marshes characterized, in order of decreasing tolerance to inundation, by Spartina foliosa , Salicorniaambigua , and Grindeliacuneifolia , and brackish marshes dominated by Scirpuscalifornicus and Typha latifolia . Suitability of habitat for shrews is determined by growth forms of the plant community, rather than species composition (Rudd 1955).
Santa Catalina Shrew
The Santa Catalina shrew (Sorexwillettti ) is probably found at least in the larger, stream-bearing canyons of Santa Catalina Island (von Bloeker 1932) and is possibly widely distributed, at least seasonally. Nothing is recorded about its habitat requirements (Williams in press).
Arizona Myotis (Bat)
The Arizona myotis (Myotisoccultus ) is most commonly associated with pine forests at 1,800–2,700 m. (6000–9000 ft.) outside California (Barbour and Davis 1969). It is known in California only from the low desert along the Colorado River (Williams in press). In most area, its roosts have been found beneath bridges and in attics of buildings (Barbour and Davis 1969), and it probably also roosts in hollows in trees and protected crevices in rocks (Williams in press).
Arizona Cave Myotis (Bat)
The Arizona cave myotis (Myotisvelifer velifer ) inhabits arid zones in the southwestern United States (Barbour and Davis 1969). Optimal foraging habitat seems to be the dense, linear stands of mesquite, tamarisk, and catclaw acacia bordering the still water and oxbow ponds along the floodplain of the Colorado River (Vaughan 1959). Preferred roost sites in California appear to be mine tunnels and caves (Stager 1939; Vaughan 1959).
Riparian Brush Rabbit
The riparian brush rabbit (Sylvilagusbachmani riparius ) is confined to dense thickets of brush such as wild rose (Rosa sp.), willows (Salix sp.), and blackberries which occur close to the San Joaquin River (Orr 1940).
Oregon Snowshoe Hare
The Oregon snowshoe hare (Lepusamericanus klamathensis ) is found primarily in riparian areas with thickets of deciduous trees such as willows and alders and in dense thickets of young conifers, particularly young firs (Williams in press).
Sierra Nevada Snowshoe Hare
The Sierra Nevada snowshoe hare (Lepusamericanustahoensis ) lives only in boreal zones, typically inhabiting riparian communities with thickets of deciduous trees and shrubs such as willows and alders (Orr 1940).
Sierra Nevada Mountain Beaver
The Sierra Nevada mountain beaver (Aplodontiarufacalifornica ) was recently discovered living along a freshwater seep near where Lee Vining Creek historically entered Mono Lake. Vegetation supported by the seep was characteristic of the herbaceous plants and woody shrubs of the riparian zone of the Great Basin sagebrushsteppe province. The area surrounding the seep predominately supported big sagebrush (Artemisia tridentata ) and rabbit bush (Chrysothamnus viscidiflorus ) (Steele in press).
Point Arena Mountain Beaver
The Point Arena mountain beaver (Aplodontiarufanigra ) primarily occupies thickets of thimbleberries on north-facing slopes (Camp 1918).
Point Reyes Mountain Beaver
The Point Reyes mountain beaver (Aplodontiarufaphaea ) is found in hillsides below 300-m. (1,000-ft.) elevation, in seepage areas overgrown with sword ferns and thimbleberries (Grinnell 1933).
Sonora Beaver
The Sonora beaver (Castorcanadensis repentinus ) inhabits slow- to moderate-flowing waters of the main channels of the Colorado River and the sloughs, canals, and oxbow lakes along the river and in the Imperial Valley (Williams in press).
Golden Beaver
The golden beaver (Castorcanadensis subauratus ) inhabits slow- to moderate-flowing streams, ponds, and lakes. Its principal requirement seems to be sufficient food, consisting of roots, bulbs, grasses, cattails, and other herbaceous plants, and bark and twigs of willows, cottonwoods, alders, and other woody plants (Grinnell etal . 1937).
Southern Marsh Harvest Mouse
The southern marsh harvest mouse (Reithrodontomys megalotislimicola ) is strictly confined to marshy areas, generally coastal salt marshes dominated by Salicornia . Adjacent weedy areas and marshes in brackish sites may also be inhabited (von Bloeker 1932).
Colorado River Cotton Rat
The Colorado River rat (Sigmodonarizonae plenus ) appears to be restricted to "isolated sections of alluvium bottom along the Colorado River" (Goldman 1928). Within this zone, it inhabits areas supporting sedges, rushes, cane, and other grasslike plants (Williams in press).
San Joaquin Valley Wood Rat
The San Joaquin Valley wood rat (Neotomafuscipesriparia ) is strictly confined to riparian communities. Nothing specific has been recorded about the habitat of this subspecies, but dusky-footed wood rats generally occur in areas supporting mixtures of trees and brush (ibid .).
White-footed Vole
The white-footed vole (Arborimusalbipes ) seems generally to be associated with small streams in forested areas and very small clearings, created by fallen timber and supporting herbaceous growth (Maser and Johnson 1967). Thickets of alder may be essential habitat for this species (Williams in press).
Yuma Mountain Lion
The Yuma mountain lion (Felisconcolor browni ) primarily inhabits the dense vegetation of the bottomland along the Colorado River; it has also been found in adjacent, rocky uplands (ibid .). Aside from adequate numbers of deer for food, the habitat requirements for this species are essentially unknown.
Discussion and Conclusions
Destruction of riparian and other wetland communities is pandemic in California. This loss and degradation is expected to increase with the increasing human population, unless measures are quickly adopted to protect remaining communities. This will be difficult to accomplish, considering the excessive human competition for limited amounts of water and the many conflicting demands placed upon riparian ecosystems. Loss of riparian and other wetland communities along the Colorado River, the low-elevation segments of the Sacramento and San Joaquin rivers, and the tidal marshes of San Francisco Bay and the Los Angeles Basin poses threats to a number of unique taxa. Biotic communities in these areas should receive priority attention in land development and resource management decisions.
Devising procedures to ensure preservation of biological diversity while permitting needed development of other natural resources is the problem before us. The present approaches are neither efficient nor cost-effective, and efforts are generally fragmented among a plethora of administrative units, resulting in duplication of efforts, gaps in coverage, competition for money and influence, and conflict.
A number of the 21 taxa treated here should be given protection as federally listed Threatened and Endangered species; this would provide for preservation of essential habitat and initiate actions directed at securing and increasing jeopardized populations. Their preservation could be accomplished most efficiently, however, by concentrating conservation efforts on their biotic communities rather than emphasizing singlespecies management. This would also provide more security to essential members of biotic communities not normally accorded protected status (e.g., lower plants, most invertebrates).
An integrated development/conservation approach which focuses upon preserving representative segments of each unique community while other resource- and land-use goals are being developed is needed. This approach would lessen the need for official listing of most of these species and save much of the money and duplication of effort now expended on management of
endangered species on a one-by-one basis. It would also ensure that resource and land development objectives are compatible with national and state goals, including the maintenance of the health and well-being of this and future generations of people.
The state should be divided into management areas based upon known and projected capabilities for resources, rate and degree of projected development, and political and administrative boundaries. We believe that resource management areas based upon major watersheds would be the most efficient approach. A task force consisting of representatives of political and resource management agencies within the management area, USDI Fish and Wildlife Service biologists, conservationists, and development interests should be established to oversee preparation of an integrated conservation/development plan for each management area. The widest possible input from the public should be sought during the formative stage of the resource area management plans.
Guidelines and procedures for implementing conservation/development plans and for amending plans in response to changing resource needs and land conditions should be established. Coordination of activities and development of goals should be accomplished at the state level by a group with a similar composition to that described for the management areas. Federal resource management agencies should set national and regional land- and resource-use goals in cooperation with the state.
Goals for use of land and resources in each management area should be defined. Unique biota and sensitive species should be identifed; resource development goals should include habitat needs of vegetation and wildlife. Harvest and use patterns of renewable resources (timber, grazing, land, wildlife, water, etc.) should be designed to optimize productivity while maintaining community diversity—this would require definition of long-term objectives for land and resource use.
Planning and implementation of long-term management of natural resources, including vegetation and wildlife, could be greatly streamlined and economized by merging land and resource management functions now fragmented among several federal agencies: Department of Agriculture (e.g., forests, soil conservation, environmental quality); Department of Commerce (e.g., marine fisheries, ocean resources, coastal management); Department of Defense (military bases and reservations); Energy Department; Department of the Interior (e.g., rangeland, minerals and mining, national parks, water, fish and wildlife). Sufficient legislation and precedents already exist, however, to implement interagency, multigovernmental programs, such as those proposed without reorganization or new legislation.
Unless actions similar to those proposed here are undertaken immediately, we are confident that populations of all 21 taxa treated here will diminish, perhaps to the point of extinction for many. These losses alone would be substantial. More alarming, however, is that these species are members of biotic communities which are rapidly diminishing. Riparian and other wetland communities are vital to most wildlife species; their degradation and loss will represent a catastrophic loss of biological diversity.
Literature Cited
Barbour, R.W., and W.H. Davis. 1969. Bats of America. 286 p. University Press of Kentucky, Lexington.
California Department of Fish and Game. 1980. At the crossroads 1980: A report on California's endangered and rare fish and wildlife. 137 p. The Resources Agency, California Department of Fish and Game, Sacramento.
Camp, C.L. 1918. Excavations of burrows of the rodent Aplodontia , with observations on the habits of the animal. University of California Publ. Zool. 18:517–536.
Goldman, E.A. 1928. Three new rodents from western Arizona. Proc. Biol. Soc. Washington 41:203–206.
Grinnell, J. 1933. Review of the recent mammal fauna of California. University of California Publ. Zool. 40:71–284.
Grinnell, J., J.S. Dixon, and J.M. Linsdale. 1937. Fur-bearing mammals of California. 2:377–777. University of California Press, Berkeley.
Hall, E.R. 1981. The mammals of North America (second edition). Volume 1 and 2. 1181 p. John Wiley and Sons, New York.
Johnston, R.F., and R.L. Rudd. 1957. Breeding of the salt marsh shrew. J. Mamm. 38:157–163.
Maser, C., and M.L. Johnson. 1967. Notes on the white-footed vole (Phenacomysalbipes ). Murrelet 48:24–27.
Orr, R.T. 1940. The rabbits of California. Occas. Papers California Acad. Sci. 19:1–227.
Rudd, R.L. 1955. Population variation and hybridization in some California shrews. Syst. Zool. 4:21–34.
Stager, K.E. 1939. Status of Myotisvelifer in California with notes on its life history. J. Mamm. 20:225–228.
Steele, D.T. in press. Mountain beaver (Aplodontiarufa ) within the sagebrushscrub habitat of Mono Basin, California. California Fish and Game.
Vaughan, T.A. 1959. A new subspecies of bat (Myotisvelifer ) from southeastern California and Arizona. University of Kansas Publ., Mus. Nat. Hist. 7:507–512.
von Bloeker, J.C., Jr. 1932. Three new mammals from salt marsh areas in southern California. Proc. Biol. Soc. Washington 45:131–138.
Warner, R.E. 1979. The California riparian study program. Phase I: Background studies and program design for phase II. 179 p. California Department of Fish and Game, Planning Branch, Sacramento.
Williams, D.F. 1979. Checklist of California mammals. Ann. Carnegie Mus. 48:425–433.
Williams, D.F. in press. Mammalian species of special concern in California. California Department of Fish and Game, Nongame Wildlife Investigations, Final Report, Project E-W-4, IV-14.1. 184 p. (draft copy). Sacramento, Calif.