XX. Marine Sedimentation
INTRODUCTION
The sea floor, being the place of accumulation of solid detrital material of inorganic or organic origin, is virtually covered with unconsolidated sediments; therefore, the study of materials found on the sea bottom falls largely within the field of sedimentation, and the methods of investigation employed are those used in this branch of geology. Twenhofel (1926) has defined sedimentation as
… including that portion of the metamorphic cycle from the separation of the particles from the parent rock, no matter what its origin or constitution, to and including their consolidation into another rock. Sedimentation thus involves a consideration of the sources from which the sediments are derived; the methods of transportation from the places of origin to those of deposition; the chemical and other changes taking place in the sediments from the times of their production to their ultimate consolidation; the climatic and other environmental conditions prevailing at the places of origin, over the regions through which transportation takes place, and in the places of deposition; the structures developed in connection with deposition and consolidation; and the horizontal and vertical variations of the sediments.
Marine sedimentation is therefore concerned with a wide range of problems, some of which are more or less unique to the sea, while others are of more general character. This discussion will deal with the first group and particular emphasis will be placed upon the “oceanographic” aspects of marine sediments. The methods of studying the character and composition of marine deposits are common to all types of sediments and, since readily available sources are cited in the text, will not be described here.
The importance of investigations in marine sedimentation is obvious when it is realized that most of the rocks exposed at the surface of the earth are sedimentary deposits laid down under the sea. In order to interpret the past history of the earth from these structures, it is necessary to determine the character of the material now being deposited in different environments. As the consolidated sediments generally contain fossils, it is of equal importance to determine the biological associations under different conditions and the character of the organic materials that may
A by-product of such studies as those mentioned above, but nevertheless of the greatest importance, is the knowledge gained concerning the history of the earth and phases of geochemistry and geology. In order to reconstruct the geological history of the earth, it is essential to obtain information on the rate of sedimentation in the oceans and to determine, either directly or indirectly, the total thickness, and hence the amount, of sediments which have been deposited in the sea. Recent research has shown that the sediments found in the deep waters of the North Atlantic are stratified, and, as stratification is related to variations in the source of material and transportational agencies, many possibilities are suggested for obtaining a better understanding of the past history of the earth. The geochemist is concerned with the chemical composition of the sediments, as they differ from the original source rocks and thus show a redistribution of the various elements, and the geologist is interested in the rate of sedimentation, as crustal movements may result from the changed distribution of mass.
In addition, many problems concerning marine sediments are of immediate significance to other phases of oceanography. To name but a few examples, the biologist is interested in the associations of organisms found in different environments on the sea bottom and the remains which may be preserved in the sediments. The occurrence of certain types of organisms has actually been deduced from their skeletal remains in the sediments before they were found living in the sea. Furthermore, the sea bottom is a zone of active breakdown of much of the detrital organic matter sinking to the bottom. From a study of the mechanisms controlling the transportation of sedimentary material it is hoped that the character of the sediments may actually be used as a measure of the water movements over the bottom.
The development of the study of marine sediments has been rapid. Sir John Murray is credited with the first intensive investigations, and his report on the Challenger material (Murray and Renard, 1891) set the pattern for many later investigations. His work was largely descriptive, identifying the various constituents and considering the source of the material. More recently, as newer methods of study—microscopical, chemical, and physical—have been developed, the character of the investigations has changed. The X-ray technique has made possible the identification of the fine-grained crystalline material which had earlier been classified as “amorphous” because it could not be recognized under the microscope. The question of precipitation and solution of calcium
CONSTITUENTS OF MARINE SEDIMENTS
Since any solid material denser than sea water and relatively insoluble may fall to the sea floor, a wide variety of substances from many sources contributes to the sediments and may be considered under six headings: (1) detrital material, largely of immediate terrigenous origin, (2) products of subaerial and submarine volcanism, (3) skeletal remains of organisms and organic matter, (4) inorganic precipitates from sea water, (5) products of chemical transformation taking place in the sea, and (6) extraterrestrial materials.
Terrigenous Material. Two processes are involved in the break-down of terrigenous rocks of either igneous or sedimentary types. These are disintegration and decomposition. Disintegration is the mechanical breakdown of the rock into smaller fragments and does not necessarily involve any change in the composition of the material. Decomposition involves chemical changes in the rock substances which are brought about by the action of water and air. Certain of the constituents are more soluble or more readily attacked and, hence, pass into solution and are carried away. The processes of weathering depend upon the character of the rock and the many aspects of the climatic conditions. Weathering depends upon the amount of rock surface exposed and, therefore, to a large degree upon the amount of disintegration which will increase the exposed rock surface (Twenhofel, 1932, 1939).
The smaller the rock fragments the more likely they are to be carried to the sea, but actually those found in the sea vary from large boulders to particles of colloidal dimensions, so small that they cannot be identified under the microscope by the ordinary petrographic methods. The material found in the marine sediments varies from easily recognizable, chemically unaltered minerals, that is, the products of disintegration, to fine material which has undergone great changes in physical characteristics and chemical composition. In the first group belong the primary minerals, quartz, mica, feldspar, pyroxenes and amphiboles, and the heavy minerals. At the other extreme are the ultimate products of
Products of Volcanism. Two types of volcanism must be considered, namely, subaerial and submarine. In both, essentially the same kinds of material may be ejected; but in the first case the volcanic ejecta will be subjected to mechanical and chemical weathering before reaching the sea. Volcanic material may be first deposited on the land and later transported to the sea by the action of running water, but the lighter and more finely divided fragments may be carried over the sea by the air. As a result of transport by winds, volcanic material may be deposited in relatively large amounts over a considerable area and, in fact, ash from single eruptions is thought to have encircled the whole world. Furthermore, pumice will float in the water for some time. Volcanic material may frequently be recognized by its physical or chemical characteristics, but it is virtually impossible to determine the percentage of highly altered material which may have arisen from this source. The following types of unaltered material may occur: lava fragments, volcanic glass, pumice, and mineral grains. The greatest amounts of volcanic material are found near areas of volcanic activity and may give rise to a characteristic type of sediment. Certain parts of the sea floor are apparently covered with basaltic lava flows of relatively recent origin, either bare or covered with a thin veneer of sediments. Submarine volcanism is probably rather common and in some localities sufficient material has accumulated to reach the sea surface and give rise to volcanic oceanic islands. Further details of oceanic bottom topography will undoubtedly show tremendous numbers of such “mountains” which do not reach the sea surface.
Remains of Organisms. The hard skeletal structures of marine organisms are important constituents of marine sediments and certain types of deposits are almost entirely composed of the calcareous (calcium and magnesium carbonate) or siliceous (hydrated silica) remains of organisms. The skeletal structures are subject to mechanical disintegration and chemical transformation, the latter generally related to solution. In marine sediments calcareous or siliceous material may exist as easily recognizable complete specimens, broken pieces still identifiable, or at the other extreme, as a mass of small crystalline fragments of uncertain origin. The calcareous type of skeletal structures may be conveniently divided into two groups, plant and animal, and the
Calcareous structures of animal origin are in general more abundant than those of plants. Many floating forms contribute to the sediments, but foraminifera are most important and deposits containing large proportions of them are known as globigerina oozes or muds. The shells of planktonic mollusks, chiefly pteropods, are also abundant in certain regions. Pelagic animals also contribute to the sediments although never to the degree that the planktonic forms do. The more resistant bony structures of fish and marine mammals are sometimes found, particularly the teeth of sharks and fish and the earbones of whales.
The benthic animals, especially those living in relatively shallow water, contribute a great deal of the calcareous material in the sediments. This is particularly true in the lower latitudes and in regions where there is little or no supply of terrigenous debris. In these cases the bottom material may be almost entirely calcareous and the remains of various groups of bottom-living animals may be represented, namely: foraminifera, corals, worms, bryozoans, brachiopods, mollusks, echinoderms, arthropods, and vertebrates.
Siliceous skeletal materials do not form so important a constitutent of sediments as calcareous structures, but in certain localities they occur in such quantities that the deposits are known by the predominant type, for example, diatom and radiolarian ooze. The only forms of plant life secreting silica are the microscopic diatoms, which grow only within the euphotic layer either as free floating planktonic forms or in shallow water as benthic organisms. Diatoms are most abundant in high latitudes and in nearshore areas where upwelling or mixing is operative, and in such localities the frustules of certain of the forms may accumulate on the bottom so that the deposits are largely made up of them. Silica is also secreted by a group of protozoa, namely the radiolarians, whose
In many ways the most important contribution of marine organisms is not the hard skeletal structures which may be preserved in the sediments, but the decomposable organic matter which is continually falling from above. Below the compensation depth (p. 779) the benthic animals and bacteria are dependent upon this supply of food from above. It may consist of entire plants or animals, faecal material, and fragments of partially decomposed organisms. In both the latter categories it may be expected that it represents the more resistant types of organic materials originally forming a part of the living organisms. This is particularly true of chitin-, cellulose-, or ligninlike substances which form part of the skeletal structures. The detrital organic matter contains carbon, hydrogen, oxygen, nitrogen, sulphur, phosphorus, and probably many other elements. The activity of the bacteria and bottom-living animals, particularly the mud-eaters, destroys much of this material and returns the constituent elements to solution in inorganic form. Marine sediments contain from virtually zero to about 18 per cent organic matter, the amount present depending upon a number of factors which will be discussed later. The deepest parts of core samples and even fossilbearing sedimentary rocks show the presence of organic matter, so that organic matter must be considered as a definite source of sedimentary material. An added interest to the problem of the supply and preservation of organic matter is that it is the source of petroleum formed from marine sediments. Although it has been implied that all the decomposable organic matter is of immediate marine origin, in certain localities such as in the Baltic Sea it is thought that a considerable proportion may be of terrigenous origin, representing the soil humus carried in by rivers.
Inorganic Precipitates. Inorganic precipitates are formed when the solubility product of some substance is exceeded. The immediate products of organic activity are excluded although the conditions necessary for precipitation of some substances may result from metabolic processes. Supersaturation may be induced by physical agencies such as temperature changes, it may be associated with the removal of carbon dioxide where photosynthesis occurs, or it may be related to changes in hydrogen-ion concentration or oxidation-reduction potential brought about by the organisms. In addition, precipitation may result from evaporation in isolated lagoons and seas.
Inorganic precipitates are never abundant in marine sediments but they may form conspicuous and diagnostically important components.
It is difficult to draw the boundaries between inorganic precipitates and those which are the direct result of biological activity and those materials which are considered to arise from the modification of minerals. Twenhofel (1939) has avoided this difficulty by considering them all as products of “chemical deposition.”
Products of Chemical Transformation. In this category are placed those substances which are formed by the interaction of sea water and solid particles. The interaction may be between the solid material and “normal” sea water or the reaction may be restricted to the interstitial water of the sediments where modified properties may exist (p. 995). It is difficult to designate many definite materials formed in this way, but undoubtedly such interactions occur, particularly when fresh unweathered materials of volcanic origin are brought in contact with sea water. Most detrital material carried to the sea has been subject to subaerial weathering, which is a continuous leaching process. In the sea the environment is much more uniform and, hence, an equilibrium may readily be established. Little is known concerning the processes of submarine breakdown of either terrigenous or volcanic material. Among those substances which may be the result of or involved in such transformations are glauconite, phosphorite, feldspar (Twenhofel, 1939), phillipsite, and the clay minerals.
Extraterrestrial Materials. In those marine sediments which accumulate at extremely slow rates, such as red clay, small black magnetic spherules and brown crystalline spherules are found. These are extremely rare and never form an appreciable part of the sediment. They were first discovered and described by Murray and Renard (1891). The black spherules are composed of iron or iron alloy and have diameters of about 0.2 mm. The brown type resemble the chondrite variety of meteorite and contain silicon. They commonly have a metallic luster and striated surface, and average about 0.5 mm in diameter.
TRANSPORTATION OF SEDIMENTARY DEBRIS
Transportation of Sediment to the Sea
Twenhofel (1932, 1939) discusses the following agencies which transport material to the sea:
Rivers and streams carrying both particulate and dissolved material.
Rainwash, slumping along river banks and sea coasts, and large scale landslides.
Shoreline erosion by waves.
Glaciers and sea ice carrying rock fragments.
Biological activity which may also increase the transport by other agencies.
Winds, which pick up large amounts of fine-grained debris from barren arid areas.
Volcanic activity, which may discharge large amounts of finegrained dust into the atomosphere.
Materials transported by the first three agencies are brought into the sea near the coast lines and the bulk is deposited near the coast, whereas material transported by the last four agencies may be carried to great distances from land before dropping to the sea bottom, and may therefore contribute significantly to the deep-sea deposits. These latter agencies will be discussed more fully.
Transportation by Ice. The transportation of sedimentary debris by ice has been and still is extremely important in high latitudes. Glaciers carry large amounts of material which they erode from the land surface, and in so doing, modify the general topography. Glaciation during the ice age has left its smark on many areas now below sea level. Certain submarine topographical features, particularly around north-western Europe and northeastern America, are attributed to glaciation. By the time of the ultimate retreat of the ice such quantities of debris had been deposited that the transportation by glaciers has placed its stamp on the character of the sediments in many localities in high latitudes.
Contemporary glacier ice carries large amounts of sediment to the sea. Such material is characterized by a great range in size, varying from enormous boulders to the finest material formed by mechanical abrasion. The character of the debris will depend on the nature of the rock formations over which the glacier passes. Icebergs carry with them the enclosed rock material and, since they may float thousands of miles before melting, this material may be deposited at great distances from its source, and pebbles and boulders may be found in otherwise pelagic deposits far from land (Murray and Hjort, 1912; Bramlette and Bradley, 1940).
Sea ice, that is, ice formed in the sea, also plays a relatively important role in the transportation of sedimentary material. This is particularly true in the Arctic seas, where in large areas of shallow water the ice may freeze to the bottom in winter. When thawing commences, the melting takes place at the surface of the ice, which will tend to rise due to its buoyancy. In this way the enclosed sedimentary material may be lifted off the bottom and, as the ice breaks up, may be transported to other localities where it will be released when the ice melts. The sea ice will tend to carry the unsorted material, including shells or other remains of organisms, from shallow water into deeper water, and will therefore give rise to anomalous accumulations of remains of organisms and organic material (Twenhofel, 1939).
Organic Rafting. A less significant amount of debris may be transported in the sea by the agency of buoyant organic material of both terrigenous and marine origin. Trees and clumps of vegetation eroded during floods or by wave action may float great distances in the sea before decomposition releases the load of imbedded rock material or until the vegetation becomes waterlogged and sinks. Leaves, branches, and even entire terrigenous plant forms are sometimes found in marine deposits far from land. Marine algae with holdfasts may be torn loose and float away, carrying rock fragments which may be deposited in deeper water (Emery and Tschudy, 1941). Benthic animals may contribute to transportation, particularly by loosening and overturning the material on the sea bottom. Man has become an agency of transportation in the sea and the effects of his activities are not uncommon in certain localities. Cinders and ash from coal-burning vessels are sometimes found in samples taken along steamer routes, as well as other refuse of such a character that it may be preserved in the sediments. Near shore, particularly in harbors, material is moved by dredging activities and, indirectly, by the construction of piers and breakwaters.
Transportation by the Atmosphere. In the dispersal of terrigenous material the atmosphere undoubtedly plays an extremely important part. The materials that are carried by the winds over the sea consist mainly of volcanic dust ejected directly into the air and of the particles that are swept up by the wind from the land surface. Wind erosion of the land is most effective in localities where high wind velocities occur and where the ground is not covered by a protective blanket of vegetation. Such areas are found in the high mountains and in desert regions and in semiarid regions where there is large-scale agricultural activity. The activities of man contribute also to the air-borne debris in many other ways which can be readily called to mind. Practically any type of organic or inorganic material may be carried by the winds if it is small enough.
Transportation of solid particles by the air is comparable to that in water, which will be discussed below. In general, the lowest wind velocities are found near the ground and material swept up from the ground must be lifted to considerable altitudes if it is to be carried over long distances. The material ejected by volcanoes is thrown directly to great altitudes where high velocities prevail and will therefore, in general, be transported for greater distances than dust which is picked up from the land surface. Consequently, volcanic material which has been at least partially air-borne is world-wide in its distribution, although in the vicinity of centers of volcanic activity it will be much more abundant and of somewhat coarser texture.
Air-brone terrigenous dust is an important contributor to marine sediments where the prevailing winds are offshore and where they have a suitable source of material. In general there will be a progressive decrease in size of the air-borne material as the distance from the source is increased, because the larger particles drop out first. Extremely fine material which may remain in suspension almost indefinitely is precipitated by rainfall or snow. The actual dispersal of sedimentary material originally air-borne may be extended by transportation in the water itself. Little is known concerning the rate of supply of air-borne terrigenous material to various localities, but data given by Twenhofel (1939) suggest that it may be relatively great.
In certain localities the amount of material transported in the air over the sea is sometimes sufficient to form dust clouds. For one such region off the west coast of Africa, Radczewski (1939) has summarized the existing knowledge of the effects of the aeolian material on the formation of the deep-sea sediments in this area. According to him the following types of grains could be identified in the dust collected from the air west of the African coast: quartz, feldspar, mica, organic siliceous remains, calcite, aggregates of small particles, and other unidentifiable material. In this locality calcite, aggregates, mica, and quartz are the most abundant. All of these materials may fall to the sea bottom, where they will be mixed with the water-borne debris and the remains of marine organisms. In sediment samples it is impossible to discriminate between the air- and water-borne material except in the case of a certain amount of the quartz grains. These are the so-called “desert quartz” grains coated with reddish iron oxides, and are characteristically aeolian material. Although the percentage of desert quartz in the original air-borne dust is not known, Radczewski determined their ratio to the total number of quartz fragments in a number of samples collected by the Meteor. These are referred to as “desert quartz numbers” and are used as indices of the proportions of aeolian material in the different samples. In general, the amount decreases away from the coast and the size of the characteristic quarz grains diminishes with increasing distance from the shore.
The size of the air-borne particles in deep-sea sediments averages less than 15 microns but larger fragments are quite abundant near the coast. Technical difficulties make it impossible to distinguish aeolian material smaller than about 5 microns.
Transportation of Sediment in the Sea
Settling Velocity. Sedimentary debris which has been transported to the sea settles through the water and is at the same time carried laterally by currents of different types. The settling velocity of a sedimentary particle depends upon its specific gravity, size, and shape, and upon the specific gravity and viscosity of the water. Before considering the settling velocity of particles in the sea it is necessary to examine the simplest case, where it is assumed that only spheres are involved. The classical equation for the settling velocity of a sphere is that developed by Stokes:
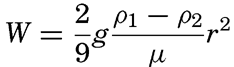
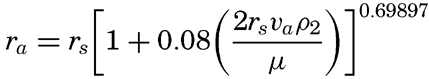
| Diameter | Settling velocity | Time to fall 10 cm | Settling velocity (m/day) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mm | Microns | Stokes’ law | Wadell | Days | Hours | Minutes | Seconds | ||
| Boulder | |||||||||
| 256 | 256,000 | ||||||||
| Cobble | |||||||||
| 64 | 64,000 | ||||||||
| Pebble | |||||||||
| 4 | 4,000 | ||||||||
| Granule | |||||||||
| 2 | 2,000 | ||||||||
| Very coarse sand | |||||||||
| 1 | 1,000 | (89.2 cm/sec) | 16.0 cm/sec | 0.6 | |||||
| Coarse sand | |||||||||
| 1/2 | 500 | (22.3 cm/sec | 7.7 cm/sec | 1.2 | |||||
| Medium sand | |||||||||
| 1/4 | 250 | (5.58 cm/sec) | 3.4 cm/sec | 2.7 | |||||
| Fine sand | |||||||||
| 1/8 | 125 | (1.39 cm/sec) | 1.2 cm/sec | 8.3 | 1040 | ||||
| Very fine sand | |||||||||
| 1/16 | 62.5 | 3482 microns/sec | 29 | 301 | |||||
| 1/32 | 31.2 | 870 | 1 | 55 | 75.2 | ||||
| 1/64 | 15.6 | 218 | 7 | 40 | 18.8 | ||||
| Silt | 1/128 | 7.8 | 54.4 | 30 | 38 | 4.7 | |||
| 1/256 | 3.9 | 13.6 | 2 | 2 | 32 | 1.2 | |||
| 1/512 | 1.95 | 3.4 | 8 | 10 | 0.3 | ||||
| 1/1024 | 0.98 | 0.85 | 1 | 8 | 41 | 0.074 | |||
| Clay | 1/2048 | 0.49 | 0.21 | 5 | 10 | 42 | 0.018 | ||
| 1/4096 | 0.25 | 0.052 | 21 | 18 | 50 | 0.004 | |||
| 1/8192 | 0.12 | 0.013 | 87 | 3 | 19 | 0.001 | |||
When mechanical analyses are made (see p. 969), the size-grade composition of a sediment is determined by sieving and by determining the settling velocity of the smaller particles. In such an analysis it is customary to employ certain physical and chemical methods for separating the individual particles which in the original sample may be cemented together or held somewhat more loosely by electrostatic or physical forces. This is particularly true of the clay and colloid material which is said to be coagulated or flocculated. Although the coarser material larger than about 15 microns may not be flocculated and will therefore settle with approximately the computed velocity, this is not true for the finer particles. Little is known concerning the state of aggregation of the clay and the colloid particles that are in suspension in the sea; however, studies by Gripenberg (1934) have shown that fine-grained material when mixed with sea water tends to flocculate into units which settle with velocity equivalent to those of quartz spheres between about 5 and 15 microns in diameter, that is, they settle between 1 m and 20 m per day. The flocculation is related to the composition of the clay minerals, particularly with respect to the exchangeable bases (p. 988) and also to the salt concentration of the water. It is probable that the flocculation is much retarded when the suspension of particles is extremely dilute. Studies of clay minerals have shown that the coagulation tends to increase the size of the units, hence to increase their settling velocity, but at the same time the coagulated particles carry with them a certain amount of the water, which reduces their effective density and therefore tends to slow them down.
A certain amount of this extremely fine-grained material may be formed in the sea, but probably most of it comes from the land, where the agencies of mechanical and chemical weathering are much more effective. If this material did not find its way to the sea floor it is obvious that the oceans would ultimately become turbid with suspended particles. Measurements of the penetration of light in the sea show that finely suspended material is present everywhere in the surface layer of the sea (p. 88), but it does not seem reasonable that the average turbidity of the water is changing. Therefore, the rate of supply of material must equal the rate of deposition. Some of the problems of submarine geology are to determine the rate of deposition, the quantity of sedimentary
Transportation of Settling Particles by Ocean Currents. The effective settling velocities of sedimentary particles probably range from less than one meter per day to many thousand meters per day. Coarse material which is brought to the sea near shore or which is released from icebergs or remains of plants at great distances from the coast will sink so fast that it is immediately deposited, but fine material with small settling velocities may be carried for considerable distances by currents. A particle 4 microns in diameter settles at a rate of about one meter per day (table 105), hence, may be carried about for many years before reaching the bottom. Such fine material may be introduced by rivers, may arise from wave action in shallow water, or may be derived from the remains of planktonic organisms. The distance to which particles can be carried depends upon their settling velocity, the velocity of the current, and in some instances upon the turbulence associated with the motion. The turbulence will lead to high values of the vertical diffusion (p. 93). This great diffusion will have no effect on the settling of very small particles if they are distributed in such a manner that the net vertical transport by diffusion is zero but, depending upon the change of concentration and the change of eddy diffusion with depth, the diffusion may lead to a net upward or downward transport of particles which may decrease or increase downward transport by settling. So far, no data are available for examination of these conditions.
Another important characteristic of the ocean currents is the presence of horizontal eddies which lead to large-scale horizontal mixing, which can be expressed as horizontal diffusion (p. 92). The horizontal diffusion is of importance near coasts where fine material is brought into the sea by rivers, wave action, wind, or other agencies. In coastal waters the great supply of fine material leads to a high concentration of suspended particles which by horizontal diffusion may be carried to considerable distances from the coast. In a steady state the amount of suspended material transported away from the coast by diffusion through a vertical plane parallel to the coast must equal the amount supplied to the water between the coast and the vertical plane minus what is deposited on the bottom inside of the vertical plane. On the basis of such considerations, Revelle and Shepard (1939) have shown how the finer material may be carried away from shore by the large-scale horizontal eddies which occur off the southern California coast. They introduced a settling velocity of 15 m per day which they consider to be that of “thoroughly coagulated suspensions.”
Transportation of Particles along the Sea Bottom. Material that settles toward the bottom is by no means always permanently
Mud flows represent movement of unconsolidated material that has accumulated on a slope and are not necessarily associated with any movement of the overlying water. Owing to instability or to a stimulus such as may be caused by seismic activity, the material begins to slide down the slope. There is some evidence that mud flows are relatively common on steep slopes and it has been suggested that they are of importance as an agency which removes accumulated sediments from submarine canyons. They are most likely to occur in areas where sediments are accumulating rapidly on relatively steep slopes, hence they may occur on the continental slope and on insular slopes. The angle of repose of unconsolidated marine sediments is not known. It is thought that mud flows can take place where the slope is only about one degree, but this does not imply that slopes of greater angle are always devoid of unconsolidated material.
Transportation by mud flows tends to break down stratification that may have developed in the deposit, and may result in the accumulation of rather coarse and unsorted material in deep water. Furthermore, mud flows may carry organisms and their skeletal remains into environments where they could not have developed, thereby leading to complications in interpretation of the environmental conditions from the study of the organic constituents of a deposit. There is very little direct evidence of the occurrence of mud flows in the seas, consequently it is as yet impossible to determine their importance as transporting agencies. The status of present knowledge is discussed by Twenhofel (1939).
In rivers the motion of individual particles along the bottom is described as sliding, rolling, and saltation (Hjulström, 1939). Sliding does not often take place, but rolling and saltation (which is a jumping motion) are common forms of transportation. Nothing is known about the extent to which such transportation takes place along the sea bottom, but certain indications can be obtained by application of results of river
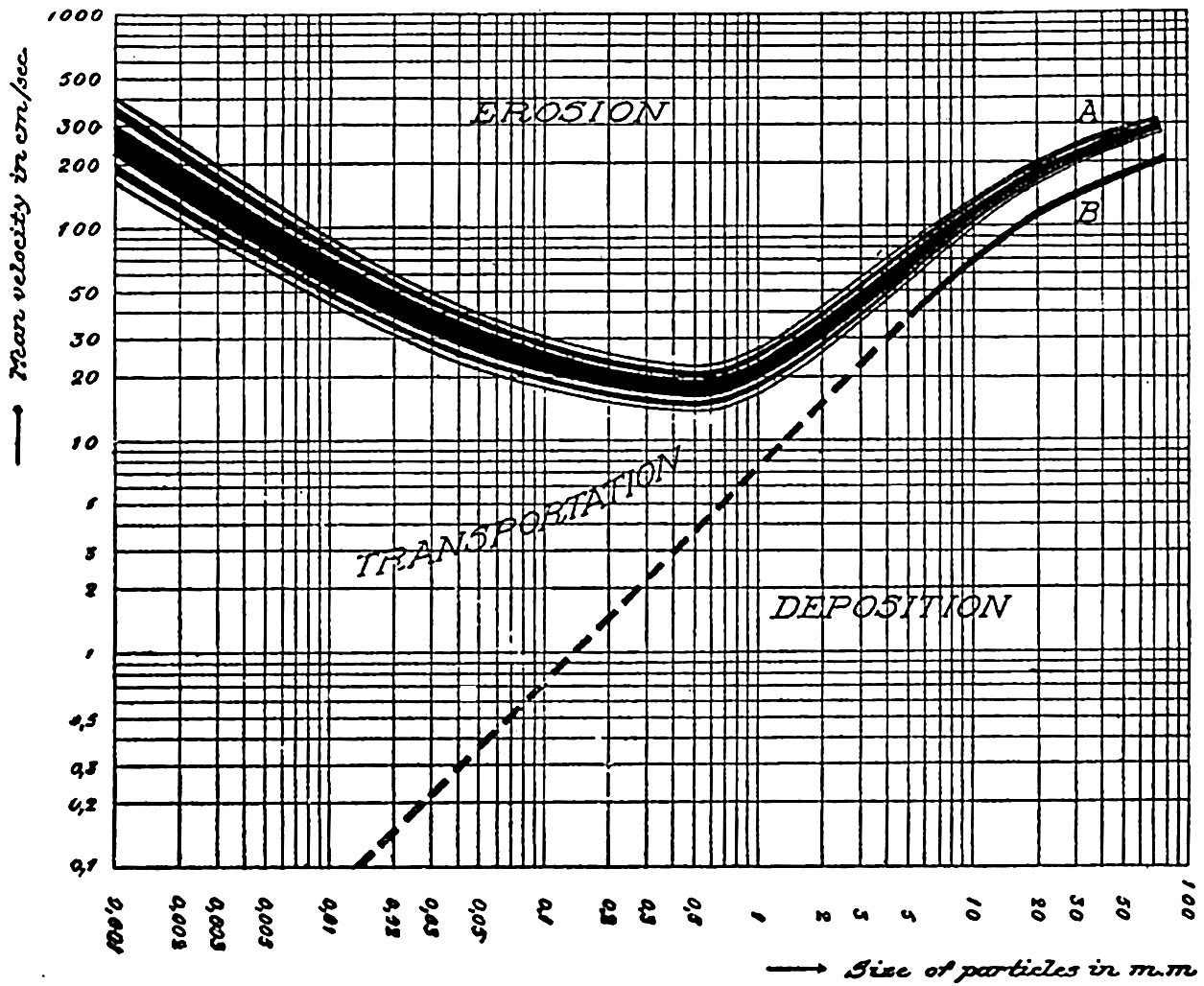
Relationship between average current velocity in a river and sediments of uniform texture showing velocities necessary for erosion, transportation, or deposition. (From Hjulström, 1939, in Recent Marine Sediments, edited by Parker D. Trask and published by the American Association of Petroleum Geologists, Tulsa, Oklahoma.)
Applying these results from rivers to the problem of transportation along the sea bottom by sliding, rolling, and saltation, it must be borne in mind that in fig. 251 the velocity represents the average velocity across a transverse profile of a river and that the velocity at the bottom is smaller. It may perhaps be assumed that the “average velocities” correspond to those occurring a few meters above the sea bottom. Taking into account the fact that high velocities near the sea bottom are common in shallow water only, it can be considered probable that eroding velocities and transportation along the bottom of coarser material, say, particles with diameters greater than 2 to 3 mm, occur only in shallow water, but that in regions of deep currents similar transportation of fine sand and silty sand may occur at depths of several thousand meters. Sediment collections made by the E. W. Scripps off the coast of California and in the Gulf of California appear to bear out such contentions except where coarse material has been found in the bottom of submarine canyons and on banks. The latter occurrence may be related to former emergence.
It should be added that on a slope alternating currents will lead to net downward transport of sediments because gravity will facilitate a downward and counteract an upward transport.
Transportation of particles in suspension near the bottom is often considered together with sliding, rolling, and saltation because the transition from one form to another is probably a gradual one. The essential difference is that material in suspension can be carried in a short time over much longer distances.
Nothing is known as to the amount or character of material in suspension near the bottom under varying conditions, but an approach to the problem can be made by application of results from laboratory studies on the characteristics of turbulent flow. Such results have been applied to the problem of transportation in rivers (Rouse, 1938), but certain modifications are necessary when dealing with transportation near the sea bottom. The basic concept is that where turbulence exists a current can carry a load of suspended particles that is determined by the condition that the downward transport by settling must equal the upward transport by eddy diffusion. The downward transport by settling equals WS where W is the settling velocity and S is the concentration of suspended particles expressed as mass per unit volume of water. The upwards transport by eddy diffusion equals 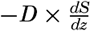 (see p. 116) where D is the coefficient of eddy diffusion and dS/dz is the concentration gradient (z is measured positive upwards). The concentration of suspended particles is therefore determined by the equation
(see p. 116) where D is the coefficient of eddy diffusion and dS/dz is the concentration gradient (z is measured positive upwards). The concentration of suspended particles is therefore determined by the equation
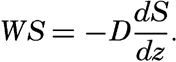
If the stability of the stratification is very small it may be assumed that near the bottom the eddy diffusion equals the kinematic eddy viscosity, that is, D = A/ρ where A is the dynamic eddy viscosity (p. 483). When dealing with currents near the sea bottom it can furthermore be assumed that in the lowest layers of the water the shearing stress is independent of the distance from the bottom and equals the stress against the bottom. This stress is (p. 479)
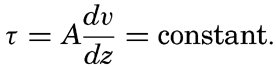
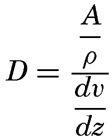
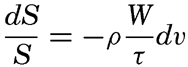
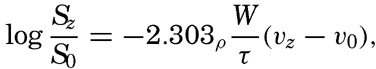
In order to make use of this general formula, specific assumptions must be made as to the variation of the eddy viscosity with increasing distance from the bottom and as to the value of the velocity at the bottom, z0. Before doing so it is necessary, however, to point out one principal difficulty that is encountered when attacking the problem in this manner, namely, that the theoretical approach leads only to relative values of the concentration of particles in suspension and not to absolute values. Actually, the mechanism which brings the particles into suspension is not clearly understood. It appears that if laminar flow exists along the bottom, that is, if the bottom is hydrodynamically smooth (p. 479), no forces are present which can thrust particles upwards. Upward thrusts probably occur only if the turbulence reaches to the very bottom, as is the case if the bottom is hydrodynamically rough. Where laminar flow is found over a smooth bottom, the velocity is always zero at the bottom and the stress exerted on the bottom varies in time only if the velocity gradient in the laminar boundary layer varies. If, on the other
Some idea as to the size or the equivalent diameter of particles in suspension can be obtained by assuming that the bottom is hydrodynamically rough. In this case (p. 479),
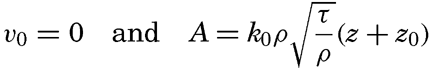
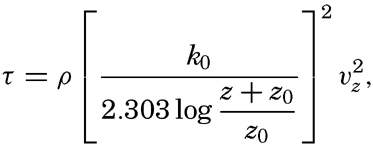
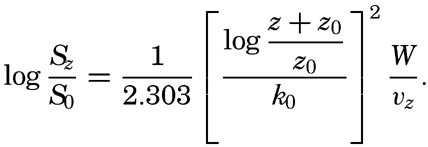
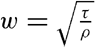 , is introduced the formula takes the simpler form
, is introduced the formula takes the simpler form
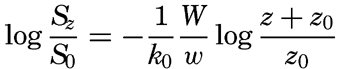
If these results are applicable to conditions near the sea bottom, one should expect that (1) very fine sand, silt, and clay can be present in suspension near the bottom, (2) the size of the particles in suspension depends upon the velocity of the current near the bottom and the roughness of the bottom, (3) the greater the velocity and the greater the roughness the larger are the suspended particles, and (4) the coarseness of the suspended material decreases rapidly with increasing distance from the bottom, provided that material of different particle sizes is present on the bottom.
In shallow water, currents of all types can bring particles into suspension. The periodic currents such as tidal currents and currents associated with seiches (p. 538) or tsunamis (p. 544) are probably the strongest; but besides these, permanent currents reaching to the bottom may exist which will carry the suspended material away. The smaller particles can then accumulate only where the deposition is greater than the transportation. This may be the case in shallow areas to which the supply of fine material is great, for example, the Mississippi Delta, or in depressions out of which no fine material is transported, for example those off the coast of southern California (fig. 264, p. 1025), or in landlocked fjords.
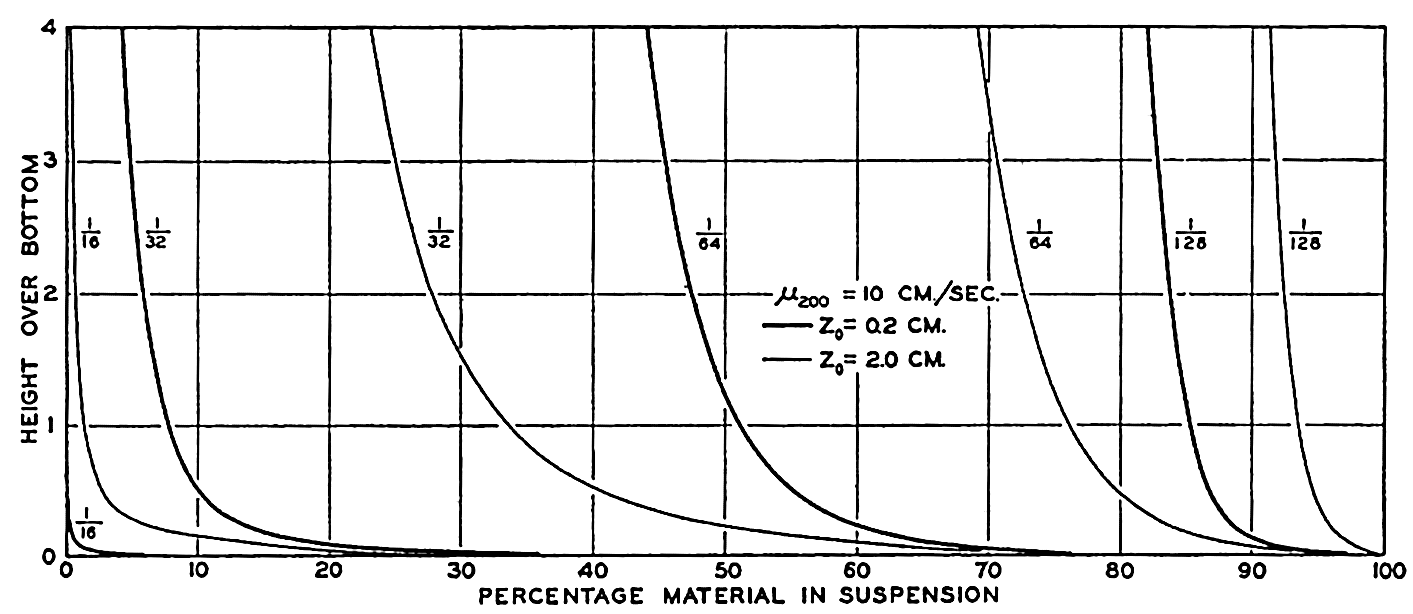
Theoretical distribution of particles of indicated diameters (1/16 to 1/128 mm) over the bottom when the velocity at 2 m above the bottom is 10 cm/sec and the roughness length is 0.2 cm and 2.0 cm.
In deep water, tidal currents are weak, but currents associated with internal waves (p. 590) may have appreciable velocities near the bottom. The permanent currents are very weak but no matter how weak they are they will transport material, which can accumulate, therefore, only where the permanent currents practically vanish, that is, in the deeper parts of ocean basins. A mechanism thus exists which will tend to sweep all finer material away from submarine ridges and peaks and will lead to accumulation of this material in the ocean basins regardless of
Effects of Transportation. The effects of transportation by the various agencies outlined above can be considered from two points of view. One can examine what happens to the shape, size, and composition of individual particles during transportation in the sea, or one can examine the net effect of transporting agencies upon the distribution of sediments. As in other aspects of sedimentation, it is impossible to do more than indicate the general character of the effects because they have not yet been studied sufficiently to afford any quantitative data. Furthermore, the physical or chemical characteristics which may be used as indices of the magnitude of transformation have not been generally applied to the study of marine sediments although the methods have been developed (Krumbein and Pettijohn, 1938).
The effects of transportation upon individual particles may modify their size, shape, and composition. In some cases the size may be increased by, say, the precipitation of calcium carbonate, but in general, transportation will tend to reduce the dimensions of solid material. Such breakdown may be the result of mechanical or chemical processes and generally both will be active. Impact or abrasion may lead to mechanical disintegration and solution, or interaction of the dissolved substances with the solid particles may tend to a reduction in size. These processes may influence not only the size of the particles but also their shape. Fracturing will reduce the size and give rise to angular fragments, whereas abrasion will generally tend to round off the sharp edges and corners. A distinction is drawn between the sphericity of particles, that is, their approach to true spherical form, and the roundness which is a measure of the smoothing away of edges and corners.
The size and shape of individual particles are not only indicative of the character of the transporting agencies and source of material but will also determine many of the properties of the sediment, for example the water content and cohesive properties of the material when wet and when dry and, for larger particles, the character of the orientation and hence the bedding of the individual grains.
The composition of the particles may be affected by the wearing away of the softer or less cohesive material by mechanical agencies or by the solution of the more soluble or more readily attacked portions by chemical processes. Such changes may be determined by examination under the petrographic microscope or by certain chemical or physical tests.
If we consider the relative effectiveness of various transporting agencies in affecting the size, shape, and composition of sedimentary material it is obvious that mechanical breakdown is most likely to occur in material moved over the sea bottom by rolling and saltation. Mechanical wear will be most rapid where particles are thrown together violently and repeatedly. In the sea such conditions are found in shallow water, where currents are strong and where the wave action extends to the bottom. The extent to which chemical weathering may act in the sea is not known, but because of the high surface: volume ratio it is undoubtedly effective on small particles and will be a function of the time of exposure rather than the character of the transporting agency. The progressive change in the character of material transported by a given agency depends upon the character of the source material, the changes in competency of the transporting agency, and the material itself and that forming the sea bottom. This general statement applies only to transportation in the water by saltation or rolling over the sea floor, as in all other agencies one or more of the variables are eliminated.
The characteristics of wind-borne material will be largely determined before it reaches the sea and are therefore outside the problem under consideration. The same is generally true of river-borne debris. Discussions of the processes of transformation associated with transportation are given by Twenhofel (1932, 1939) and Russell (1939), and the methods of determining the properties of individual grains are given by Krumbein and Pettijohn (1938).
MASS PROPERTIES OF MARINE SEDIMENTS
The mass properties of sediments are the summation of those of the component grains. Because of the variety in size and composition of the ultimate particles, the mass properties are not clear-cut and in some cases statistical methods must be used to express them. Three of the mass properties, namely, color, texture, and composition, are used for the classification of marine sediments. The advantages of such a system are that preliminary examination in the field, by visual examination and from the feel of the sample when rubbed between the fingers, enables an experienced observer to classify the sample correctly. Besides being useful to designate the type of sediment, these properties give certain indications as to the source of the constituent material (particularly the grains large enough to be recognized with a hand lens), the effective transporting agencies, and the conditions in situ.
Color of Marine Sediments. The color of a recent marine sediment depends upon the following factors: (1) whether the sample is wet or dry, (2) the primary color of the larger grains, (3) the state of division (size) of the constituent particles, (4) the state of oxidation or reduction of the iron, and (5) the amount of decomposable organic matter.
Because the color may be affected by the moisture content and the oxidation-reduction potential may be modified by bacterial activity after the sample is collected, the color should be noted soon after the material has been obtained. In order to eliminate as far as possible the subjective element in judging color when an accurate estimate is desirable, a standard color chart such as that prepared by Ridgway (1912) should be employed.
The color of the individual grains is particularly important in sediments containing a relatively large proportion of material coarser than silt. Finely divided material may have an “apparent” color which depends upon the state of division. In many ways the oxidation-reduction potential in the sediment is probably the most important factor controlling the color. When conditions are such that the potential is oxidizing for iron, reddish or brown oxides are formed. This is the condition which will normally prevail in the presence of free oxygen. When there is a relatively abundant supply of organic material, bacterial activity may use up all the available oxygen and establish highly reducing conditions. If hydrogen sulphide is produced, black iron sulphides may be formed. Intermediate stages in the oxidation or reduction of the iron may possibly produce the greenish and bluish colors characteristic of many near-shore deposits. Although the state of oxidation or reduction of the iron has been considered as the most important factor in determining the color, particularly of fine-grained sediments, the problem is not yet solved, as the sediments tend to retain their color even when treated in various ways in the laboratory.
Marine sediments vary from white through gray to black, with the addition of various amounts of yellow, red, blue, or combinations of these colors. White sediments and, in general, those of light colors are relatively coarse-grained and composed of quartz or limestone. The grays may be due to the presence of black minerals or authigenic iron and manganese grains. Black deposits are typical of stagnant conditions that may prevail in basins, fjords, and other isolated environments.
Coarse-grained sediments will generally show the color of the constituent grains. As the greatest part of the sea floor is covered with fine-grained sediments, it is of interest to note the character of the material brought to the sea. River-borne debris is generally yellow, red, or brown, and in areas where the deposition is rapid, as off the mouths of large rivers, the sediments are essentially the same color as the source material and are often referred to as red muds. Along most coasts where the deposits are predominantly terrigenous, the sediments are generally green or olive green. Hence, there seems to be some change in the color of the terrigenous material that is probably associated with the oxidation-reduction potential in the environment of deposition. Further offshore the greenish or bluish muds pass gradually into the
Both the absolute and the relative rates of deposition of decomposable organic matter and of organic skeletal structures and inorganic debris may be important in determining the color of a deposit. It is a characteristic property of many terrigenous deposits that the upper few millimeters are of a browner tint than the underlying material. This may be due to the fact that the supply of oxygen is much greater at the surface and that the oxidation-reduction potential is not as low as it is in the buried material. Lamination or stratification of the sediments in core samples may frequently be recognized by color differences. Such structures can generally be confirmed by studies of the texture or of the organic-matter content. The darker bands usually contain more organic matter.
The organic-matter content of marine sediments varies from essentially zero to more than 15 per cent. Samples containing large quantities may actually be darkened by the presence of the black or brownish organic detritus. Fox and Anderson (1941) have shown that organic pigments can be extracted from certain terrigenous muds.
Color is therefore a useful character in classifying marine sediments because it tells something of the source of the material, particularly of the coarse-grained portions, and of the conditions of deposition. If the depth and distance from shore are known, certain conclusions may be drawn as to the relative and absolute rates of deposition of decomposable organic matter and of the inert constituents and as to the oxidation-reduction potential prevailing in the sediments.
Texture of Marine Sediments. Depending upon their texture, marine sediments may be subdivided into gravels, sands, silts, muds, clays, or intermediate types (p. 972). The proportions of larger grains may be estimated visually or judged by rubbing the material between the fingers. An experienced observer is able to classify a sample in this way very easily. When an accurate knowledge of the particle-size distribution is required, laboratory tests must be made by methods such as those described by Krumbein and Pettijohn (1938) and by Gripenberg (1939b).
The particles in a marine sediment may cover a wide range in size. In general, there are no sharp limits to the sizes of the constituent grains, and as a result it is necessary to employ certain conventionalized methods for the discussion or for the graphical presentation of data of this type. In practice, it is impossible to determine the size of each individual grain
The data on size-grade distribution may be graphed in various ways. The two most common forms are histograms and cumulative curves. Because of the range in particle size usually encountered and for other reasons, it is convenient to plot histograms and cumulative curves showing the percentage weight frequency against the logarithm of the equivalent diameter. In figs. 254 and 255 (pp. 980, 981), comparable data are represented in these two ways. By convention, the smaller grade sizes are always on the right-hand side of the diagram. The heights of the columns in the histograms show the weight frequency or percentage represented by the individual size grades. The cumulative curve is obtained by successive addition of the material in the different grades and shows directly the percentage by weight of material greater or less than any designated size.
Krumbein and Pettijohn (1938) and Krumbein (1939) have discussed the statistical significance of size-grade distribution data. Certain values which can be obtained directly from the cumulative curve for an individual sample are commonly used to characterize the general size and the sorting of the sediment. These are:
The median diameter (M2) which represents the diameter which in the individual sample has 50 per cent by weight of material of smaller and larger equivalent diameters.
The first (Q1) and third (Q3) quartile diameters. The first quartile diameter has 25 per cent smaller and 75 per cent larger diameter and the third quartile has 75 per cent smaller and 25 per cent larger. The difference in the quartile diameters indicates the range covered by the middle 50 per cent of the material, but tells nothing of the coarser and finer quarters.
The sorting coefficient (S0) is a measure of the degree of sorting and is defined as
The value of S0 will approach unity as the material becomes more uniform in size. Trask (1932) found that values of S0 less than 2.5 indicated well-sorted material with about 3.0 as the average for marine sediments.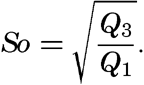 ― 971 ―When S0 is greater than 4.5, the sediment is considered to be poorly sorted.
― 971 ―When S0 is greater than 4.5, the sediment is considered to be poorly sorted.The asymmetry of the distribution about the median is designated as the skewness (Sk). The skewness may be defined as
When the size-frequency distribution curve is symmetrical the value of Sk is unity, but the values may range from less than unity to larger figures which indicate that the distribution is skewed either towards the finer or the coarser sizes.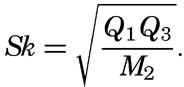
The statistical expressions given above are those recommended by Krumbein and Pettijohn (1938) and may differ from somewhat similar terms employed by other workers.
It will be seen that the median and the first and third quartile diameters and the sorting coefficient and the skewness are introduced in order to obtain simple figures to express certain features of the size-grade distribution. It is unfortunate that these expressions do not take into account the coarse and fine material lying beyond the limits of the first and third quartiles. This drawback is particularly applicable as concerns the coarser material, which may be diagnostic as to the effective transporting agencies. The finer material is not of such interest as it is more cosmopolitan in its distribution.
Composition of Marine Sediments. The composition of a sediment may be presented in various ways and a wide variety of techniques may be employed to determine it. In general, “composition” is used to indicate one of the mass properties, it may be the size-grade distribution discussed above, the chemical composition, or the percentage of different types of organic remains or minerals. On the other hand, interest may be centered upon some fraction of the sample, such as the heavy minerals or the decomposable organic matter, or upon some single chemical element. The composition is of interest because it is a way of determining the sources of the various constituents and the proportions in which they are present. Furthermore, it offers a means of determining the effects of chemical weathering and of identifying authigenic minerals.
In the field the composition of the larger grains may be determined by visual examination which may tell whether they are predominantly organic and if so, of what type, or whether they are minerals of volcanic, terrigenous, or authigenic nature. From such observations, in combination with the color and texture, the sediment may be classified. In the laboratory, examination under the microscope makes it possible to identify many more of the grains down to sizes of approximately two microns. Below this grade it is necessary to employ chemical analyses, X-ray analyses, or some other method to determine the character and
Complete chemical analyses are difficult and are rarely carried out. Much more frequently, determinations of one or more diagnostic elements are made, for example, of calcium carbonate, or organic nitrogen as a measure of the organic matter.
CLASSIFICATION OF RECENT MARINE SEDIMENTS
Marine deposits are subdivided into two major groups, termed pelagic and terrigenous. The pelagic deposits are those found in deep water far from shore and may be predominantly either organic or inorganic in origin. Pelagic deposits are light-colored, reddish or brown, fine-grained and, generally, they contain some skeletal remains of plankton organisms. Benthic forms are generally rare. The inorganic deposits are referred to as red clay and the organic deposits as oozes. The terrigenous deposits are found near shore and generally contain at least some coarse material of terrigenous origin. They cover a wide range in depth and a great variation in color, texture, and composition. They are known as sands, silts, muds, or intergrading types, depending upon their texture. Although they may have median diameters as small as certain pelagic deposits, the terms clay and ooze are never applied to terrigenous deposits. Nearshore sediments may contain remains of plankton forms but, in addition, skeletons of benthic organisms may be relatively abundant. In certain regions the deposits may be almost entirely made up of the fragments of certain calcareous benthic forms.
Pelagic deposits are classified in the following way:
Inorganic deposits. Those which contain less than 30 per cent of organic remains are known as red clay.
Organic deposits. Those which contain more than 30 per cent of material of organic origin are known as oozes. This class is further subdivided into:
Calcareous oozes. These contain more than 30 per cent calcium carbonate, which represents the skeletal material of various plankton animals and plants. The calcareous oozes may be further divided into three types, depending upon a characteristic type of organism present in the sediment, namely:
Globigerina ooze, in which the calcium carbonate is in the tests of pelagic foraminifera.
Pteropod ooze, containing conspicuous shells of pelagic molluscs.
Coccolith ooze, containing large numbers of coccoliths and rhabdoliths that form the protective structures of the minute Coccolithophoridae.
― 973 ―Siliceous oozes. These are pelagic deposits which contain a large percentage of siliceous skeletal material produced by planktonic plants and animals. The siliceous oozes are subdivided into two types on the basis of the predominance of the forms represented, namely,
Diatom ooze, containing large amounts of diatom frustules, hence, produced by plankton plants.
Radiolarian ooze, containing large proportions of radiolarian skeletons formed by these plankton animals.
The classification of terrigenous deposits is not so satisfactory as that of the pelagic sediments. A number of systems have been suggested, but the character of the deposits depends so much upon local conditions that no one system has very wide application. Relative to the pelagic deposits, terrigenous sediments cover a small percentage of the sea floor, and since they show a wide range in properties within a relatively short distance from the coast the occurrence of transitional types is more of a problem. As pointed out in the discussion of mass properties, color, texture, and composition form the basis of the classification. Hence it is desirable to indicate the terms applied to terrigenous deposits rather than set up any definite outline of classification.
The color of terrigenous deposits may range from white to black with the addition of blue, yellow, or red, or mixtures of these. In general, sediments of the shelves and slopes are dark in color, ranging from green or brown to blue. The color depends so much on the local environment that it may vary a great deal in a relatively short distance over the bottom, or with depth in the deposit.
Terrigenous deposits are generally coarser than those found in the deep sea, although certain exceptions do occur. The texture will depend not only upon the effective transporting agency but also upon the character of the source material. The following terms have been suggested by Revelle (personal communication) to designate the texture of terrigenous deposits:
Sand. More than 80 per cent of the material coarser than 62 microns in diameter.
Silty sand. Between 50 per cent and 80 per cent coarser than 62 microns.
Sandy silt. More than 50 per cent coarser than 5 microns and more than 20 per cent coarser than 62 microns.
Silty mud. More than 50 per cent coarser than 5 microns and less than 20 per cent coarser than 62 microns.
Clayey mud. Less than 50 per cent coarser than 5 microns.
By definition (p. 957) the sands may be subdivided into very coarse (2000–1000 microns), coarse (1000–500 microns), medium (500–250 microns), fine (250–125 microns), and very fine (125–62 microns). If all the material is within the limits of 62 and 4 microns the term silt may be used. It will be noted that the term mud was not listed in table 105, since it is restricted to terrigenous sediments containing particles with a wide range in size.
The composition is concerned with the character and, hence, with the source of the constituent materials. On this basis terrigenous sediments may be divided into organic and inorganic types. The terms foraminiferal, coral, diatomaceous, or other specific terms may be applied when a single type of organic remains is prominent in the sediment, and if no one form is conspicuous the general terms calcareous or siliceous may be used with the understanding that they indicate material of organic origin. Predominantly inorganic types of sediments may be expected to be related to the character of the material supplied to any area, and in this case no special term need be applied. However, the presence of mineral grains of certain types or sizes may make it desirable to add a term indicating either the source or mode of transportation. Thus, glacial may be used for sediments containing material deposited by glaciers and icebergs, volcanic when there is a large amount of pumice, volcanic ash, and glass in the deposit. Mica is sometimes a readily recognized constituent and if it is abundant the term micaceous may be introduced. In certain areas authigenic glauconite is sufficiently abundant to warrant use of the term glauconitic.
When following the above system of terminology a trinomial nomenclature may be used to describe terrigenous deposits. For example, a sediment from near a continental coast may be a green diatomaceous silty mud, or one from the shelf of an oceanic island may be a gray calcareous sand.
DISTRIBUTION OF PELAGIC SEDIMENTS
General Features of Distribution. Figure 253 shows the distribution of the various types of pelagic sediments. The representation is generalized partly to avoid confusion and partly because of the incomplete knowledge as to the types of sediments found in many parts of the oceans. Any such presentations of the distribution of pelagic sediments are modified versions of maps originally prepared by Sir John Murray and his associates. Further investigations have changed the boundaries but have not materially affected the general picture. The figure has been prepared from the most recent sources available. The distribution of sediments in the Indian Ocean is based on a map by W. Schott (1939a), that in the Pacific Ocean is from W. Schott in G. Schott (1935), with some revisions based on Revelle's studies of the samples collected by the Carnegie (Revelle, 1936). The data for the Atlantic have been drawn from a number of sources, since no comprehensive map has been prepared for many years. The Meteor material has been described by Correns (1937 and 1939) and Pratje (1939a). Thorp's report (1931) on the sediments of the Caribbean and the western North Atlantic was used for those areas, and Pratje's data (1939b) for the South Atlantic were supplemented by those of Neaverson (1934) for the Discovery samples. The distribution in the North Atlantic is from Murray (Murray and Hjort, 1912).
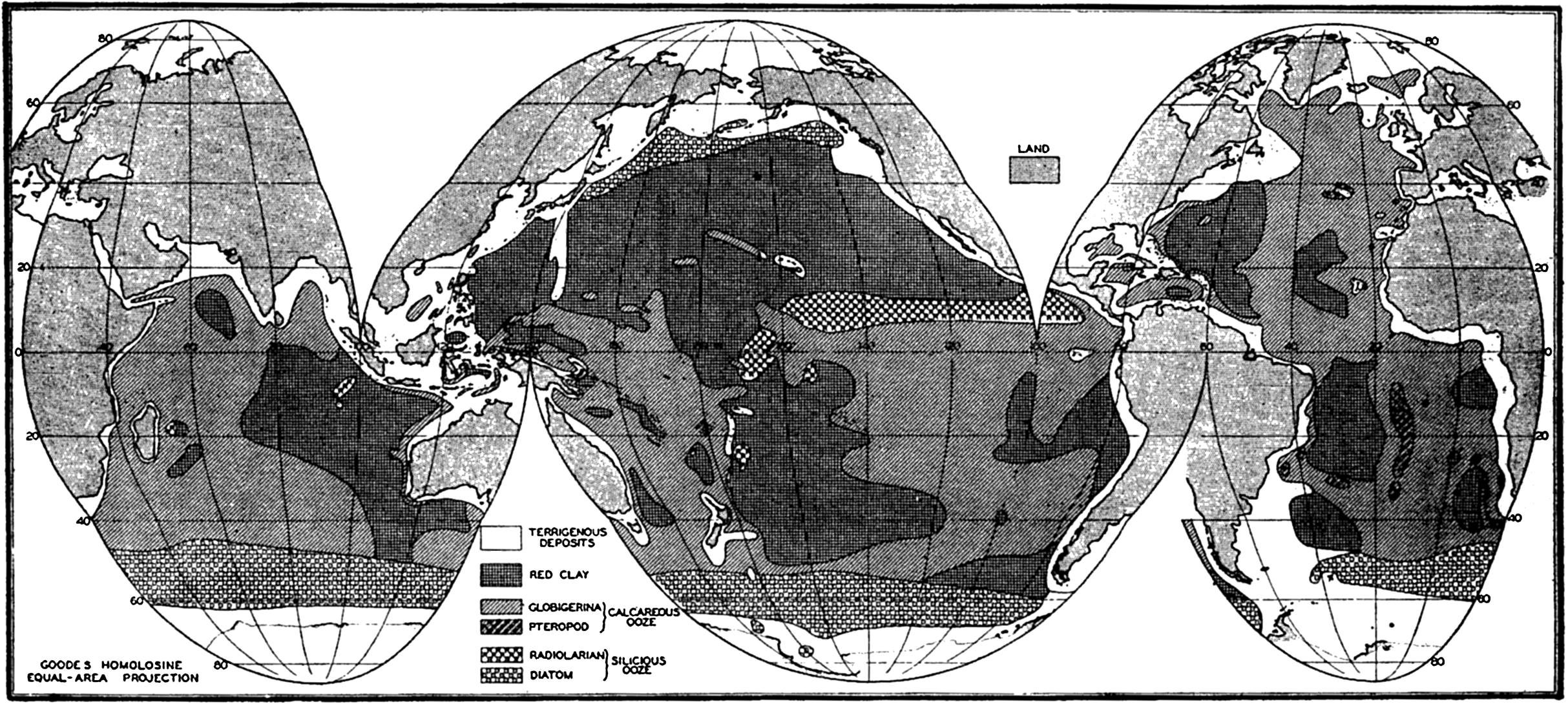
Distribution of the various types of pelagic sediments.
One type of shading has been used for all of the calcareous sediments and another for the siliceous sediments. Unless the symbol P is shown to indicate that the area is covered with pteropod ooze, it is to be understood that the calcareous sediment is globigerina ooze. The siliceous organic sediments are indicated as D for diatom ooze and R for radiolarian ooze. The unshaded areas of the oceans and seas are covered with terrigenous sediments.
Various features of the distribution of pelagic sediments should be pointed out:
Pelagic sediments are restricted to the large ocean basins.
Red clay and globigerina ooze are the predominant types of deposits.
Diatom oozes are restricted to a virtually continuous belt around Antarctica and a band across the North Pacific Ocean.
Radiolarian ooze is almost entirely limited to the Pacific Ocean, where it covers a wide band in the equatorial region.
Pteropod ooze occurs in significant amounts only in the Atlantic Ocean.
The width of the area of terrigenous sediments depends upon a number of factors such as the depth and the supply of material, but it should be noted that in general it is more extensive in high latitudes. The North Polar Basin and the seas adjacent to the northern Pacific and Atlantic Oceans are covered with terrigenous sediments. As will be shown later, the terrigenous sediments of lower latitudes are largely composed of calcareous remains of benthic organisms in contrast to those of higher latitudes, which are chiefly made up of mineral fragments.
Although no depth contours are shown in fig. 253, comparison with chart I will show that the distribution of red clay and calcareous oozes is restricted to those portions of the ocean floor with moderate or great depths.
The boundaries between different types of sediments are not distinct, since one form will graduate into another with interfingering where the topography is irregular. However, a glance at the figure will show that the marginal belts are small compared to the tremendous areas
― 977 ―of readily classified sediments, and it is for this reason that the system of classification can be considered valid.
Area of Ocean Bottom Covered by Pelagic Sediments. In table 106 are given the areas covered by the different types of pelagic sediments. The values were obtained from fig. 253. Pelagic sediments cover 268.1 × 106 km2 of the earth's surface, that is, 74.3 per cent of the sea bottom. The calcareous oozes (47.7 per cent), notably globigerina ooze, are the most extensive, with red clay (38.1 per cent) next in importance among the pelagic deposits. Siliceous oozes cover only 14.2 per cent of the total area.
| Atlantic Ocean | Pacific Ocean | Indian Ocean | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Area | % | Area | % | Area | % | Area | % | |
| Calcareous oozes: | ||||||||
| Globigerina | 40.1 | 51.9 | 34.4 | |||||
| Pteropod | 1.5 | |||||||
| Total | 41.6 | 67.5 | 51.9 | 36.2 | 34.4 | 54.3 | 127.9 | 47.7 |
| Siliceous oozes: | ||||||||
| Diatom | 4.1 | 14.4 | 12.6 | |||||
| Radiolarian | 6.6 | 0.3 | ||||||
| Total | 4.1 | 6.7 | 21.0 | 14.7 | 12.9 | 20.4 | 38.0 | 14.2 |
| Red clay | 15.9 | 25.3 | 70.3 | 49.1 | 16.0 | 25.3 | 102.2 | 38.1 |
| 61.6 | 100.0 | 143.2 | 100.0 | 63.3 | 100.0 | 268.1 | 100.0 | |
The percentages of the total area of pelagic sediments in the three oceans covered by the major types of sediments are as follows:
| Sediment | Indian Ocean | Pacific Ocean | Atlantic Ocean |
|---|---|---|---|
| Calcareous oozes | 54.3 | 36.2 | 67.5 |
| Siliceous oozes | 20.4 | 14.7 | 6.7 |
| Red clay | 25.3 | 49.1 | 25.8 |
| 100.0 | 100.0 | 100.0 |
It will be seen that calcareous deposits predominate in the Indian and the Atlantic Oceans, but that in the Pacific Ocean, which is somewhat deeper, red clay is the most extensive. Of the total areas covered by the three major types of sediments the percentage distribution in the three oceans is as follows:
| Sediment | Calcareous oozes | Siliceous oozes | Red clay |
|---|---|---|---|
| Indian Ocean | 26.9 | 33.9 | 15.7 |
| Pacific Ocean | 40.6 | 55.3 | 68.7 |
| Atlantic Ocean | 32.5 | 10.8 | 15.6 |
| 100.0 | 100.0 | 100.0 |
The Pacific Ocean, because of its great size, contains the largest percentage of all of the three types and actually over 50 per cent of the siliceous oozes and red clay.
Depth Range of Pelagic Sediments. Depth is generally considered as one of the factors controlling the distribution of the different types of marine sediments. According to Murray's classification, deep-sea sediments are restricted to depths greater than about 200 m, and in general pelagic sediments are found only at considerably greater depths. Although there is some difference in the depth distribution in the three oceans, data are not comparable, and the following values for globigerina and pteropod oozes and red clay are from Murray and Chumley (1924), representing the results of studies made on 1426 samples from the Atlantic Ocean. The values for diatom and radiolarian oozes are from Andrée (1920).
| Sediment | Samples | Depth (m) | ||
|---|---|---|---|---|
| Minimum | Maximum | Average | ||
| Globigerina ooze | 772 | 777 | 6006 | 3612 |
| Pteropod ooze | 40 | 713 | 3519 | 2072 |
| Diatom ooze | 28 | 1097 | 5733 | 3900 |
| Radiolarian ooze | 9 | 4298 | 8184 | 5292 |
| Red clay | 126 | 4060 | 8282 | 5407 |
Although the ranges overlap, indicating that factors other than depth control the distribution of pelagic sediments, it can be seen that radiolarian ooze and red clay are characteristic of depths greater than 4000 m, whereas the calcareous sediments and diatom oozes are generally restricted to the lesser depths.
MASS PROPERTIES OF DEEP-SEA SEDIMENTS
Color of Deep-sea Sediments. The significance of color has been considered above, and in the system of classification it was pointed out that pelagic deposits are characterized by light colors or red or brown
The colors of the various types of pelagic sediments are as follows:
Globigerina ooze. Milky white, rose, yellow, or brown. Near land it may be dirty white, gray, or blue.
Pteropod ooze. White to light brown with reddish, pink, or yellow tinge.
Diatom ooze. Yellowish, straw, or cream colored. Dirty white when dry. Darker near land.
Radiolarian ooze. Red, chocolate brown, or straw colored.
Red clay. In North Atlantic, brick red, but in South Pacific and Indian Oceans, chocolate brown. Graduates into “blue” near shore.
As pointed out in the section on classification, the color of terrigenous deposits is extremely variable. Murray and his co-workers established various types of terrigenous sediments to which the names “blue,” “green,” and “red” muds were applied. Unfortunately, the color was not the only factor which placed a given sample within one of these groups, therefore the anomalous situation developed that a green- or brown-colored mud would be classified as a “blue mud.”
Texture of Deep-sea Sediments. The texture of pelagic sediments varies a great deal, depending largely upon the proportions of organic remains. Pteropod and globigerina oozes, containing little or no fine material, which occur on topographic highs actually are sands if texture alone is considered. At the other extreme are the fine-grained clays. It is convenient, as in the discussion of color, to consider the textural properties of the three extreme types of pelagic sediments, namely, red clay, diatom ooze high in organic remains, and globigerina ooze largely made up of foraminiferal skeletons. Intermediate types representing organic oozes diluted with clay will of course fall within the limits described by these extreme types. Coarse-grained mineral fragments are never abundant except under special circumstances.
The following data have been selected as examples. The average of five globigerina oozes from the Pacific Ocean collected by the Carnegie were analyzed by the pipette method by Revelle (1936). These samples
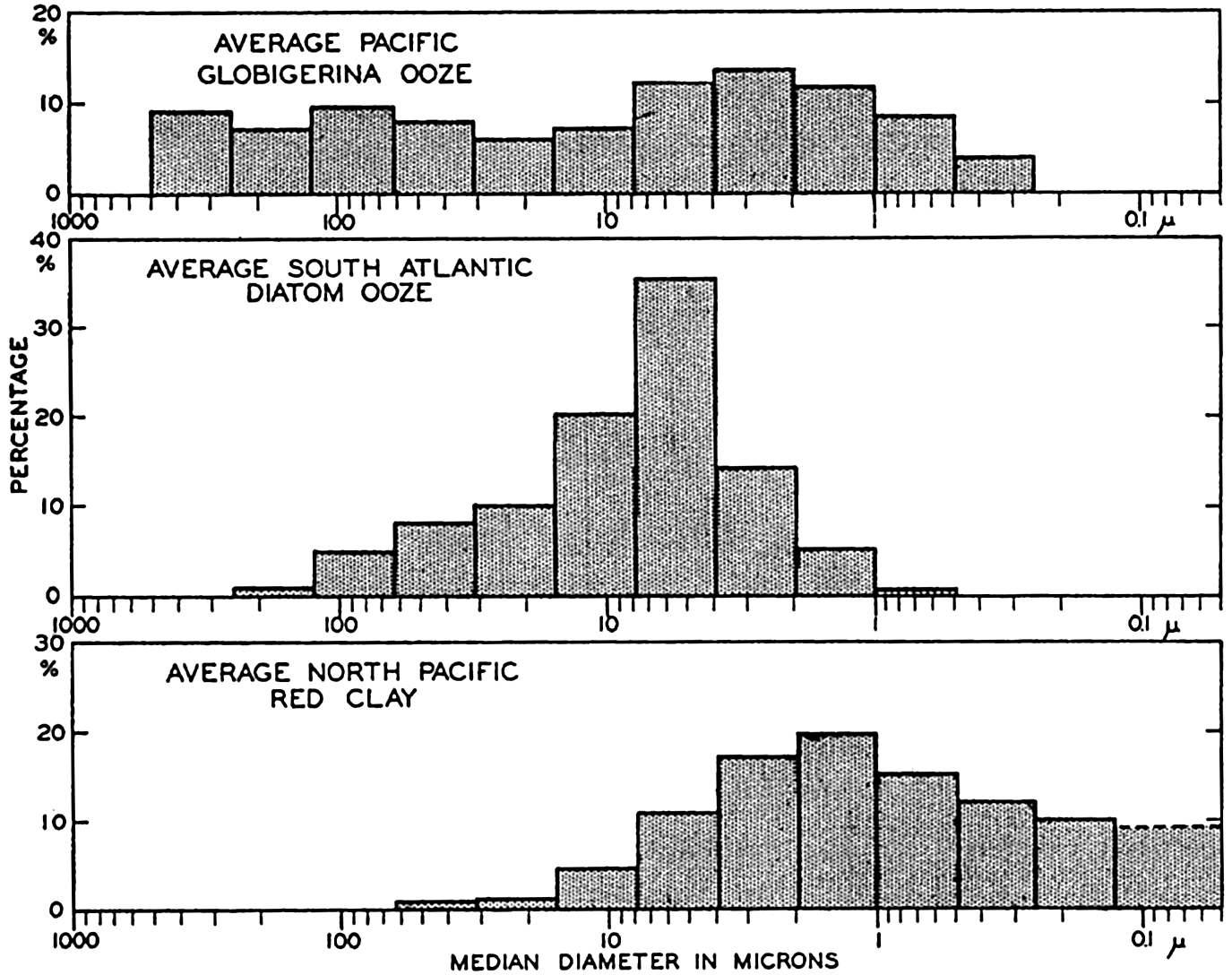
Distribution of particle size (equivalent diameter) in pelagic sediments represented by histograms (same data as in fig. 255).
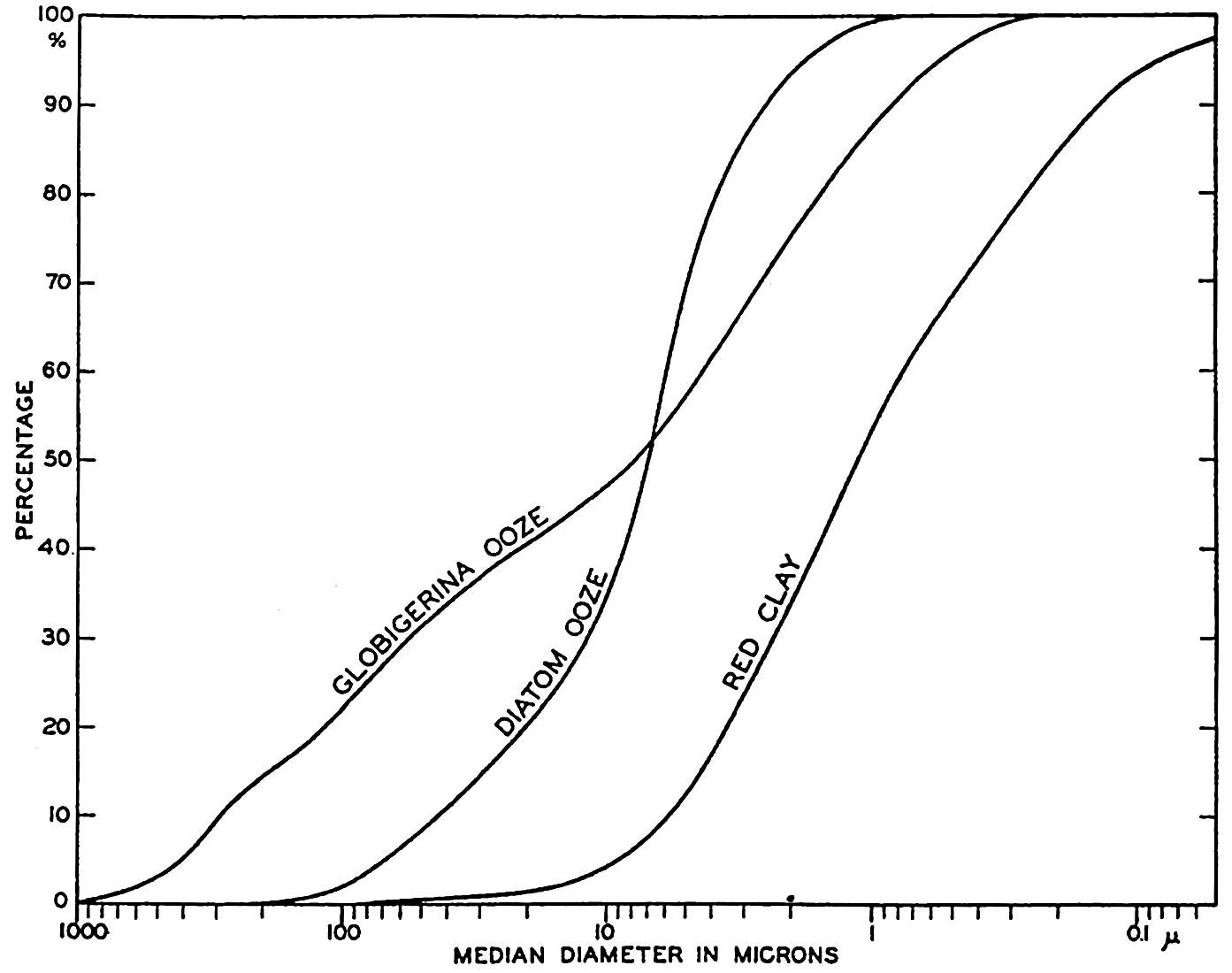
Distribution of particle size (equivalent diameter) in pelagic sediments represented by histograms (same data as in fig. 254).
| Sediment | Sand % | Silt % | Clay % | M2 μ | Q1 μ | Q3 μ | So | Sk |
|---|---|---|---|---|---|---|---|---|
| Globigerina ooze | 28.7 | 33.5 | 37.8 | 7.8 | 2.0 | 82.0 | 6.4 | 4.58 |
| Diatom ooze | 6.0 | 88.0 | 6.0 | 7.0 | 4.4 | 15.0 | 1.85 | 3.63 |
| Red clay | 0.0 | 17.3 | 82.7 | 1.1 | 0.35 | 2.8 | 2.83 | 0.94 |
The median diameter for the globigerina ooze does not represent the size of the complete tests which would mostly have diameters greater than 62.5 microns. It will be noted that the histogram shows several maxima. The histograms of globigerina oozes show two maxima characteristically, one in the coarser grades which corresponds to the relatively complete tests, and another in the fine grades. Revelle (1936) has shown that corresponding maxima are found in the CaCO3 content of the different size grades. The maximum in the small grades is due to the presence of small crystals of CaCO3 (calcite) which are believed to arise from the mechanical disintegration of the foraminiferal tests. Correns (1939) considers that the fine-grained calcareous particles result from the destruction of the albuminoid (pseudochitin) cement which binds together the carbonate crystals in the shells. More than 50 per cent of the material finer than 2 microns may be calcareous. The data for the diatom ooze show that the bulk of the material is in the silt grades with the largest fraction between 7.8 and 3.9 microns. Because of their shape and structure, radiolarian and diatom frustules will have equivalent diameters smaller than their true dimensions. Revelle (1936) shows in photomicrographs many entire radiolarians of dimensions larger than 250 microns, and the material between 250 and 125 microns is almost entirely composed of diatom frustules and smaller radiolarians.
From the foregoing discussion it may be seen that the pelagic deposits contain material of diameters ranging from a millimeter or more down to the clay fraction with diameters chiefly less than one micron. Excluding the more or less exotic volcanic material and such rare constituents as manganese nodules, sharks’ teeth, and so forth, it is obvious that the coarser fractions of true pelagic deposits are entirely composed of skeletal remains, whereas at the other extreme there is the inorganic clay fraction. The size-grade composition of a given sample may fall anywhere between these extremes and have only one maximum or several maxima, depending upon the character of the source materials and the relative proportions in which they are deposited.
Physical Composition of Deep-sea Sediments. The character of the grains larger than about two microns can generally be determined by microscopic examination. The organic remains can be identified, and with the aid of the microscope and various techniques of the petrographer the mineral grains may also be recognized. The fine material, such as that predominating in red clays, cannot be studied in this way because of the small size of the individual particles. For many years this material was considered as “amorphous,” but the development of X-ray methods for determining crystal structure and the application of various physical and chemical tests have established the identity of the clay minerals.
In the examination of sediment samples the mechanical analysis is usually made first and various convenient size grades are separated.
Organic constitutents in deep-sea sediments comprise remains of virtually every type of marine organisms possessing hard skeletal structures. The various oozes may consist almost entirely of the remains of one type of planktonic organisms; on the other hand virtually all types of organisms may be found in any one sample, although the total may not make up more than a small percentage of the deposit. The latter situation may prevail in red clays. Foraminifera, both planktonic and benthic, have been studied most extensively, and the species encountered and their distribution are fairly well known. Much less information is available concerning the species of diatoms, radiolarians, and other forms encountered in marine deposits. In many reports on the physical composition of marine sediments no attempt has been made to classify the organic remains beyond reporting the percentages of calcareous and siliceous structures. Revelle (1936) has tabulated the general organic constituents in 1156 pelagic sediment samples studied by Sir John Murray and his associates. These data are given in table 108, taken from Revelle. In it are shown the maximum, minimum, and average percentages of calcium carbonate, planktonic and benthic foraminifera, other calcareous remains, siliceous remains, and the mineral grains of diameters greater and less than 0.05 mm (50 microns) in the various types of pelagic sediments. If the extreme cases are selected, as in the case of the texture, it may be seen that red clays may contain 100 per cent of minerals less than 0.05 mm, whereas calcareous oozes may contain more than 98 per cent of calcareous remains, and siliceous oozes 90 per cent of siliceous remains. However, most samples represent mixtures of two or more of these basic types of constituents in variable proportions. The average percentage of various organic and inorganic materials can be obtained from table 108.
Calcareous structures are in general more abundant than siliceous organic remains. The various groups of pelagic and benthic organisms which may contribute to the sediments have been listed elsewhere (p. 950). Both planktonic and benthic foraminifera are found in marine sediments. Cushman (1928) lists 25 species of planktonic foraminifera. Revelle found 23 species in the deposits of the Pacific Ocean, Throp (1931) 19 species in the deposits of the western North Atlantic, and W. Schott (in Correns et al, 1937) 21 species in the Equatorial Atlantic.
| Physical composition | Red clay (%) | Radiolarian ooze (%) | Diatom ooze (%) | Globigerina ooze (%) | Pteropod ooze (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| (C) | (M) | (C) | (C) | (V) | (C) | (M) | (M) | ||
a Only in two exceptional cases; the usual maximum is not more than 5 per cent. | |||||||||
b Only in one exceptional case. | |||||||||
c Includes finely divided remains of siliceous organisms. | |||||||||
| CaCO3 | Maximum | 28.8 | 29.0 | 20.0 | 36.3 | 24.0 | 96.8 | 97.2 | 98.5 |
| Minimum | 0 | 0 | tr | 2.0 | 0 | 80.2 | 30.0 | 44.8 | |
| Average | 6.7 | 10.4 | 4.0 | 23.0 | 2.7 | 64.5 | 64.7 | 73.9 | |
| Planktonic foraminifera | Maximum | 27.0 | predominant part of CaCO3 | 80.0 | 95.0 | 75.0 | |||
| Minimum | 0 | 25.0 | 15.0 | 15.0 | |||||
| Average | 4.77 | 8.8 | 3.1 | 3.1 | 53.1 | 58.9 | 34.7 | ||
| Benthic foraminifera | Maximum | 3.0 | 10.0 | 10.0 | |||||
| Minimum | 0 | present | 0 | tr | |||||
| Average | 0.6 | 0.6 | .1 | 1.6 | 2.1 | 2.1 | 3.6 | ||
| Other calcareous remains | Maximum | 6.3 | 31.8 | 26.0 | 57.0 | ||||
| Minimum | 0 | present | 1.2 | tr | 15.8 | ||||
| Average | 1.3 | 1.0 | .8 | 3.2 | 9.2 | 3.7 | 35.5 | ||
| Siliceous remains | Maximum | 5.0 | 80.0 | 60.0 | 90.0 | 10.0 | 15.0[a] | 20.0 | |
| Minimum | 0 | 30.0 | 20.0 | 40.0 | 4.0 | tr | tr | ||
| Average | 2.4 | 0.7 | 54.4 | 41.0 | 73.1 | 1.6 | 1.7 | 1.9 | |
| Texture of mineral particles | |||||||||
| >.05 mm, diameter | Maximum | 20.0 | 60.0[b] | 5.0 | 25.0 | 40.0 | 50.0[b] | 50.0[b] | 20.0 |
| Minimum | 1.0 | tr | 1.0 | 3.0 | 1.0 | 1.0 | tr | tr | |
| Average | 5.6 | 2.4 | 1.7 | 15.6 | 8.4 | 5.3 | 5.1 | 4.7 | |
| <.05 mm, diameter | Maximum | 100.0 | 67.0[c] | 27.9[c] | 34.0 | 66.6 | 69.3 | 41.8 | |
| Minimum | 31.0 | 17.0[c] | 12.5[c] | 9.0 | 1.2 | 1.2 | tr | ||
| Average | 85.4 | 86.5 | 39.9[c] | 20.4[c] | 15.8 | 30.6 | 28.5 | 19.6 | |
| Number of samples averaged | 70 | 126 | 9 | 5 | 16 | 118 | 772 | 40 | |
According to the studies of W. Schott (1937) the benthic foraminifera constitute less than 1 per cent of the total number of foraminifera in about 50 per cent of the samples examined. In very few cases do they exceed 25 per cent of the total number of tests. The highest percentages occur in samples of intermediate CaCO3 content. This is in agreement with Revelle (1936), who found the greatest number of species in samples of intermediate carbonate content.
W. Schott (1937) has introduced the “foraminiferal number” as a measure of the abundance of relatively entire tests. He found that most of the unbroken tests were in the size grade between 2 to 0.2 mm diameter. By count he determined that 1 g of this material contained 23,300 tests. The foraminiferal number is then obtained by multiplying the fraction of the material in this size grade, less the percentage of material other than foraminiferal tests, by 23,300. From this it can be seen that a sample composed entirely of foraminifera in this grade would have a foraminiferal number of 23,300. The highest value obtained by Schott was 12,700. Much of the calcium carbonate is present as smaller tests or as broken fragments, hence the foraminiferal number is not directly proportional to the CaCO3. The relationship obtained by Schott is shown in fig. 256. Correns (1939) has suggested that the term globigerina ooze be restricted to samples with foraminiferal numbers greater than 6000, which would indicate that at least one quarter of the sediment consisted of unbroken foraminiferal tests in the size grade of diameter between 2 and 0.2 mm. As can be seen from fig. 256, such samples would contain more than 60 per cent CaCO3.
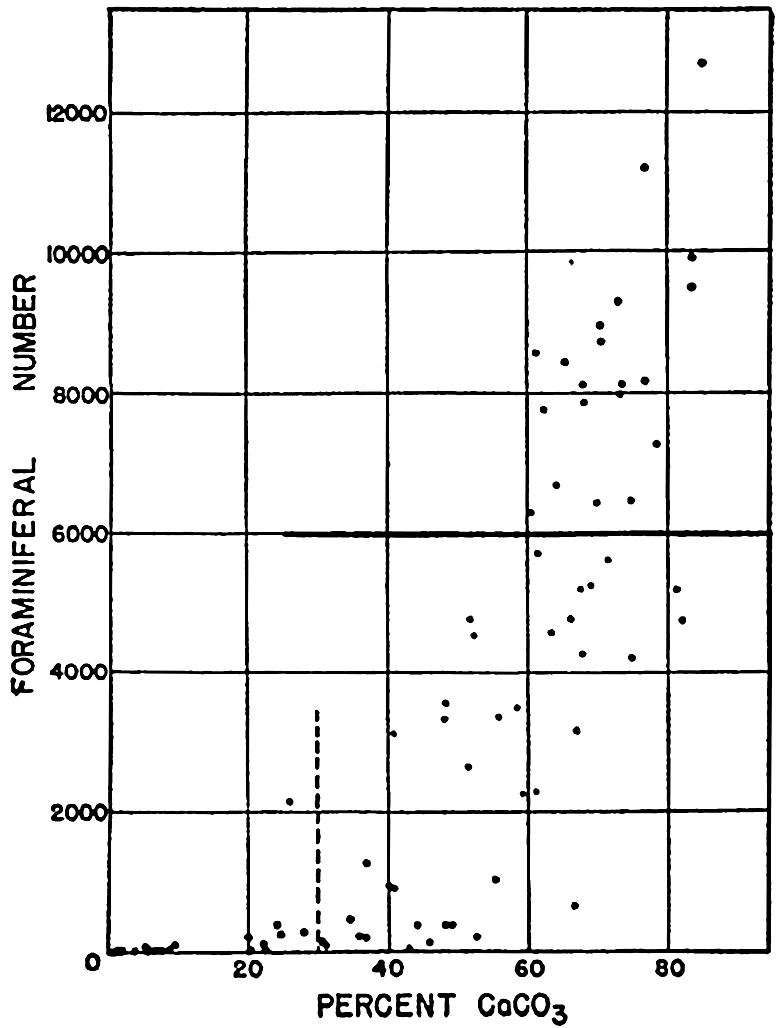
Relation of the foraminiferal number to the calcium carbonate content in sediments from the Equatorial Atlantic Ocean. (From Correns, 1939, in Recent Marine Sediments, edited by Parker D. Trask, and published by the American Association of Petroleum Geologists, Tulsa, Oklahoma.)
Because of their relatively large size, pteropods and heteropods are conspicuous constituents of certain calcareous oozes from the shallower depths in tropical latitudes. However, they never constitute more than
Calcareous remains of other types of pelagic and benthic animals may be found in pelagic deposits but they are never abundant. Echinoderms, annelids, ostracods, and polyzoa are among the benthic forms commonly present.
Calcareous structures of plant origin are among the important constituents of the pelagic deposits. Fragments of the larger calcareous algae (Lithothamnion and Corallina) are often found near land, and in the open ocean the remains of the microscopic Coccolithophoridae may be very abundant, particularly in the tropical and subtropical parts of the Atlantic Ocean. These pelagic forms are so delicate that the entire skeletons are rarely found but, instead, only the tiny plates of which they are built up. The oval plates called coccoliths and the rod-shaped fragments called rhabdoliths, which arise from the coccosphere and the rhabdosphere, may constitute as much as 74 per cent of the material between 10 and 2 microns in diameter and may form about 15 per cent of the material of finer grades. According to Correns (1939), the calcareous plant remains never form more than 13 per cent of the entire sample.
The inorganic constituents in deep-sea sediments are shown in table 109 (Revelle, 1936), giving the frequency with which the principal minerals were reported by Murray and his co-workers in the various types of pelagic deposits. These records are based on examination of the material larger than 0.05 mm and do not include the smaller particles referred to by Murray as “fine washings.” Table 108 gives the maximum, minimum, and average content of “minerals” and “fine washings” in the various types of deposits and, as can be seen from the average values, the larger grains rarely represent more than a small percentage of a pelagic sediment. Coarse terrigenous material must be rare except in regions where ice or the atmosphere are effective, because of the lack of adequate transporting agencies. Volcanic material, except pumice which will float for some time and the fine dust which may be air-borne, will also be restricted to areas of volcanic activity. The authigenic materials, on the other hand, are not directly dependent upon transporting agencies but are related to the environment prevailing in and over the sediment.
Although means have been available for many years for the identification of mineral grains, it is only recently that it has been possible to obtain any adequate idea as to the character of the fine material. Murray considered it as amorphous, that is, noncrystalline, and from the available data decided that red clays and the clay fraction of all pelagic deposits were formed on the sea bottom by the decomposition of volcanic material. It was also suggested that a certain “insoluble” fraction of calcareous and siliceous skeletons might contribute to this fraction of the deposit.
| Percent of samples analyzed in which sustance is recorded | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Principal inorganic constituents | Red clay (%) | Radiolarian ooze (%) | Diatom ooze (%) | Globigerina ooze (%) | Pteropod ooze (%) | ||||
| (C) | (M) | (C) | (C) | (V) | (C) | (M) | (M) | ||
a Always listed as hornblende, except for one reference to glaucophane, in a blue mud, and to actinolite, in a globigerina ooze. | |||||||||
b Includes all references to monoclinic feldspar. | |||||||||
c Includes all references to triclinic feldspar. | |||||||||
d Includes three references to green mica in blue and green muds. | |||||||||
e Includes one reference to bronsite in globigerina ooze. | |||||||||
f Does not include clay minerals, iron hydroxides, or colloidal silica. | |||||||||
g May be allogenic in part. | |||||||||
| 1. Allogenic minerals | |||||||||
| Amphibole[a] | 44 | 19 | 10 | 100 | 60 | 50 | 36 | 60 | |
| Chlorite | |||||||||
| Epidote | 3 | ||||||||
| Feldspar, undifferentiated | 76 | 19 | 90 | 60 | 90 | 73 | 38 | 50 | |
| orthoclase[b] | 40 | X | |||||||
| sanidine | 10 | 18 | 10 | ||||||
| plagioclase[c] | 29 | 80 | 20 | 10 | |||||
| Garnet | 1 | 20 | X | ||||||
| Magnetite | 89 | 23 | 100 | 80 | 29 | 40 | |||
| Mica, undifferentiated | 27 | 40 | 10 | 40 | 6 | 26 | 64 | 70 | |
| white mica | |||||||||
| black mica[d] | 10 | 20 | |||||||
| Olivine | 7 | 40 | 16 | 10 | |||||
| Pyroxene, undifferentiated | X | ||||||||
| enstatite | X | ||||||||
| hypersthene[e] | |||||||||
| augite | 61 | 13 | 30 | 40 | 12 | 70 | 12 | 40 | |
| Quartz | 30 | 58 | 80 | 80 | 42 | 68 | 70 | ||
| Tourmaline | 4 | 20 | X | ||||||
| Zircon | 4 | 20 | X | ||||||
| 2. Authigenic minerals[f] | |||||||||
| Analcite | 10 | ||||||||
| Glauconite | 9[g] | 14[g] | 20 | 6 | 11 | 13 | |||
| Manganese grains, nodules | 79 | 43 | 70 | 31 | 6 | ||||
| Palagonite | 31 | 9 | 40 | 20 | 8 | 30 | 40 | ||
| Phillipsite | 14 | 40 | X | ||||||
| Phosphate | X | ||||||||
| 3. Rock fragments, etc. | |||||||||
| Crystalline, sedimentary | 7 | 60 | 12 | 9 | |||||
| Volcanic, glass | 64 | 23 | 70 | 80 | 70 | 60 | 65 | ||
| lapilli, scoria | 16 | 10 | 20 | ||||||
| pumice | 49 | 8 | 20 | 40 | 30 | 40 | 30 | ||
| rock fragments | |||||||||
| 4. Magnetite, other (cosmic) | |||||||||
| spherules | 11 | 30 | X | ||||||
The development of X-ray methods for determining the crystal structure has opened up a new field in the study of fine-grained material and the application of this technique (Revelle, 1936, Mehmel 1939) has shown that the fine clayey material is largely crystalline. Methods of identification based on X-ray spectroscopy (Mehmel, 1939) and upon certain physical and chemical tests such as the temperature-dehydration characteristics and the base-exchange capacity have made it possible to identify many of the constituents of the clay fraction. The general problems in this field have been reviewed by Grim (1939) and Kelley (1939). Revelle (1936) and Correns et al (1937) have shown the presence of quartz, calcite, and other minerals, as well as a group of substances generally known as “clay minerals” in the fine fraction of marine sediments.
Clay minerals have the following characteristic properties. Within any group there is a range in chemical composition due to isomorphous replacement. They generally contain a large amount of water partly bound as water of crystallization and partly by surface and interlattice adsorption. The platelike crystal units are mechanically weak and easily broken down and may be ruptured by the process of dispersion. Many of the clay minerals possess the property of base-exchange. In base-exchange, cations in a solution may enter the crystal structure and replace a stoichiometric equivalent of another ion which passes into the solution. For this reason it is possible to have hydrogen, sodium, potassium, calcium, and other clays, where these elements are the replaceable cations. The physical properties of such clays depend upon the replaceable cation or cations. For example, sodium clays are sticky, impermeable, and readily dispersed, whereas calcium clays are relatively granular, porous, and difficult to disperse.
The replaceable cations can be arranged in a series with decreasing replacing power:
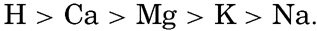
Grim (1939) considers that there are three important groups of clay minerals as well as certain miscellaneous types that do not belong to the major groups. Under natural conditions mixtures of clay minerals usually occur.
Kaolinite Group. The chief member is kaolinite which has the formula (OH)8Al4Si4O10. Other rarer forms also belong in this group.
Montmorillonite Group. The clay mineral of this name has the formula (OH)4Al4Si8O20(xH2O). The others in this group are beidellite, nontronite, and saponite. Magnesium is usually found in montmorillonite; in beidellite the SiO2:Al2O3 molecular ratio is 3 instead of 2; in nontronite, iron replaces some of the aluminum and in saponite, magnesium replaces some of the aluminum.
Illite Group. This group has the general formula
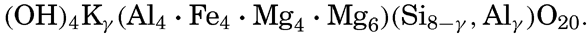
Miscellaneous Clay Minerals. In this category are placed halloysite [(OH)8Al4Si4O10] and hydrated halloysite [(OH)16Al4Si4O6]. The noncrystalline mutual solutions of silica, alumina, and water, that may also contain some bases and for which the term allophane has been proposed, are placed in this group.
The data on the physical and chemical properties of clay minerals have been assembled by Grim (1939). The base-exchange capacity, expressed as milliequivalents per 100 g, is greatest for montmorillonite (60 to 100), intermediate for illite (20 to 40), and least for kaolinite (3 to 15). Little or nothing is known about the other types.
The clay minerals are known to result from the weathering of rocks under the action of water and its dissolved substances, particulary carbon dioxide. Plants and the products of organic decomposition also contribute to the breakdown of the rock fragments. The evidence now available indicates that the character of the residual minerals depends more upon the conditions or environment of weathering than upon the type of rock attacked.
The investigations of Mehmel on the Meteor samples and of Revelle on the Carnegie samples have shown the presence of a variety of clay minerals. The sediments of the Equatorial Atlantic contained kaolinite, montmorillonite, and hydrated halloysite, and a muscovitelike clay. Revelle obtained essentially the same results, and in addition found that certain deposits of the South Pacific contained appreciable amounts of noncrystalline siliceous material. From this evidence it would appear that at least part of the clay fractions are not formed on the sea bottom but represent the products of subaerial weathering carried into the sea
There is no sharp change in mineralogical composition or properties in passing from known terrigenous deposits to the pelagic deposits.
There is no progressive change in the mineralogical constituents with depth in cores from pelagic deposits. If submarine weathering was active, changes with time should occur which would be reflected in the cores.
The variety of fine-grained minerals indicate a number of sources, as would be found in terrigenous debris.
As stated elsewhere (p. 958), fine-grained material, particularly in dilute suspensions, is not necessarily flocculated by mixing with sea water, hence can be transported for great distances in the sea.
Although the present evidence does not preclude the formation of some clay minerals from volcanic debris on the sea bottom, there is considerable evidence which indicates that much of the clay fraction found in marine deposits, both nearshore and pelagic, is of terrigenous origin. The detection of this finely divided material in the water and the determination of its distribution in the ocean waters will be the conclusive argument in deciding upon the source of the red clay and the nonorganic fraction of the pelagic oozes.
Many of the clay minerals in the sediments possess the property of base-exchange. The question of whether or not any exchange takes place when terrigenous clays come in contact with sea water is unsettled. Kelley and Liebig (1934) found that clay minerals treated with sea water attained an equilibrium in which magnesium formed the principle replaceable base, followed by sodium, calcium, and potassium. In the sea water the order of abundance of these cations is sodium, magnesium, calcium, and potassium (p. 176). The high proportion of magnesium in the clays is due to its high replacing power, while sodium takes second place because of its abundance in the water. Revelle (1936) found that the base-exchange capacity of three clay samples was relatively high (27, 34, and 39 milliequivalents per 100 g) and that the size fractions less than 1.5 microns had even higher values. In the two cases reported these were 53 and 58 milliequivalents per 100 g. Magnesium was the most abundant replaceable cation. In the whole samples, the ions were in the order magnesium, calcium, sodium, potassium, but in the material of diameters less than 1.5 microns the sodium exceeded the calcium.
It should be pointed out that leaching with pure water or treatment with any salt solution, such as those used as dispersing agents, will tend to bring about certain changes in the exchangeable cations in the clay minerals. Hence, chemical analyses or studies of the base-exchange properties must be made on unwashed samples of the sediments.
Chemical Composition of Pelagic Sediments. As pointed out above, the composition of pelagic deposits covers a wide range and no two specimens will ever be identical in physical or chemical composition. Therefore it is desirable to turn our attention once more to the three extreme types of material commonly found in pelagic deposits, namely, calcareous remains, siliceous skeletal structures, and the fine clay material.
In table 48 (p. 231) are given examples of the chemical composition of various types of skeletal structures. Revelle (1936) analyzed foraminiferal tests carefully separated from five samples of globigerina ooze. The maximum, minimum, and average amounts of the various determined constituents are given in table 110. The average CaCO3 content is 96.2 per cent. According to Revelle the high iron content may be due to changes occurring after deposition. The composition of the siliceous skeletal material may be taken to be hydrated silica (SiO2xH2O). Hence, the organic constituents will yield two extreme types of deposits which may be either virtually all CaCO3 or all SiO2. Traces of many other elements are undoubtedly represented, and there are many minor anomalies in the composition of skeletal material. For example, among the Radiolaria there are forms that secrete strontium carbonate and others that have skeletons of complex silicates, but these will not materially affect the general picture.
| Constituents | Maximum | Minimum | Average |
|---|---|---|---|
* Only two determinations. | |||
| SiO2 | 0.72 | 0.08 | 0.47 |
| Al2O3[*] | 1.35 | 0.22 | 0.78 |
| FeO,Fe2O3 | 1.68 | 0.51 | 1.08 |
| MgO | 0.16 | 0.10 | 0.13 |
| CaO | 54.52 | 53.12 | 53.82 |
| CO2 | 43.10 | 41.69 | 42.37 |
| Ignition loss[*] | 0.87 | 0.78 | 0.72 |
| 99.47 | |||
When considering the composition of the fine-grained clay material the question arises as to whether or not it is possible to consider a single type of composition or whether there are a number of varieties. The available data indicate that the first approach is a valid one. In table 111 are given three analyses of “pure” red clays. These have been adjusted to a basis as being free from salt, decomposable organic matter, calcium carbonate, and water. The analysis by Steiger (Clarke, 1924) was made on a composite sample prepared by Sir John Murray from
| Constituents | Composite of 51 samples (Steiger) | Average of 10 Pacific Ocean samples (Revelle) | Atlantic Ocean sample (Correns) | Average of igneous rocks (Clarke, 1924) |
|---|---|---|---|---|
| SiO2 | 54.48 | 55.95 | 53.27 | 59.14 |
| TiO2 | 0.98 | 0.83 | 0.98 | 1.05 |
| Al2O3 | 15.94 | 18.48 | 23.74 | 15.34 |
| Cr2O3 | 0.012 | 0.055 | ||
| Fe2O3 | 8.66 | 8.16 | 2.65 | 3.08 |
| FeO | 0.84 | 3.39 | 3.80 | |
| Ni,CoO | 0.039 | 0.025 | ||
| MnO | 0.31 | 0.124 | ||
| MnO2 | 1.21 | 0.83 | ||
| MgO | 3.31 | 2.95 | 0.38 | 3.49 |
| CaO | 1.96 | 0.45 | 2.48 | 5.08 |
| SrO | 0.056 | 0.022 | ||
| BaO | 0.20 | 0.06 | 0.055 | |
| K2O | 2.85 | 3.35 | 2.36 | 3.13 |
| Na2O | 2.05 | 1.32 | 2.27 | 3.84 |
| V2O3 | 0.035 | 0.026 | ||
| As2O3 | 0.001 | tr. | ||
| MoO3 | tr. | |||
| P2O5 | 0.30 | 0.18 | 0.02 | 0.30 |
| CuO | 0.24 | 0.015 | ||
| PbO | 0.008 | 0.002 | ||
| ZnO | 0.005 | 0.005 | ||
| ZrO | 0.14 | 0.04 | ||
| H2O | 7.04 | 7.30 | 8.15 | 1.15 |
| Total % | 100.00 | 100.00 | 100.00 | 99.77 |
| Total Fe as Fe2O3 | 9.59 | 8.16 | 6.18 | 7.31 |
| Total Mn as MnO2 | 1.21 | 0.83 | 0.38 | 0.15 |
THE ENVIRONMENT OF DEPOSITION
Samples of sedimentary rocks can be stored for years without altering their properties, but this is not true of samples of recent sediments, in which marked changes may take place after collection, particularly if they are allowed to dry out. These changes are chiefly associated with activity of organisms and the loss of water. Organic processes subsequent to collection of samples will generally not follow the pattern existing on the sea floor because of the changed environment. Increased bacterial activity will tend to modify the organic-matter content, the oxidation-reduction conditions, and the pH. The loss of water by evaportation may change the textural characteristics of the sediment, since sediments which have been dried do not disperse as completely as fresh samples (Correns, et al, 1937). Furthermore, the changing salt concentration of the interstitial water which will accompany evaporation may result in base-exchange and precipitation of salts.
The temperature prevailing in marine sediments can be closely estimated from that of the water immediately adjacent to the bottom and depends upon depth, latitude, season, and circulation, and in basins upon the surrounding topography. The temperature probably has its greatest effect through its influence upon the character and activities of the organisms living in or on the sediment. Hydrostatic pressure, which is a function of depth, is of no significance and has no effect either upon the organisms or upon the water content or porosity of the sediment. The water content can be considered an important characteristic that is part of the environment of the sediments on the sea floor. The water content, which must be determined on carefully preserved samples, is commonly reported as the weight per cent of the sediment. However, if reported as volume per cent (assuming a mean grain density of 2.6), it is an expression of the porosity. Trask (1932) discussed the water content of sediments and Krumbein and Pettijohn (1938) have given many references to studies of the porosity of sedimentary rocks. The porosity, hence the water content, depends upon the texture, shape, and sorting of the particles in the sediment. Furthermore, the compaction must be taken into account. Recently deposited material has a greater pore space and water content than that which has “aged” or which has had additional material deposited on top, that is, has been subjected to pressure. In fine-grained material, particularly the clays and the so-called colloid fraction, the effect of surface adsorption must be considered. Trask fractionated a marine sediment into a number of size grades and determined the water content of the freshly settled material (table 112). He found that the water content increases rapidly in materials smaller than about 20 microns in diameter. After standing or after the superposition of additional material the water content decreases
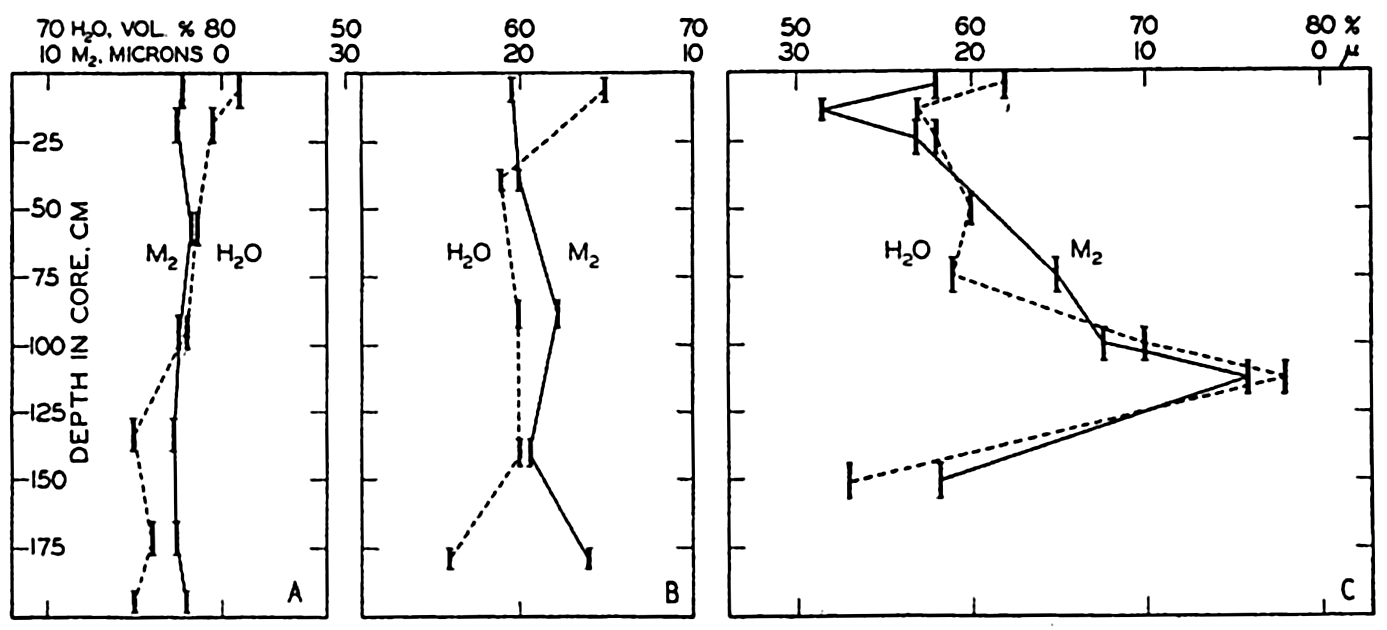
Water content and median diameter in core samples of terrigenous sediments obtained off the California coast. Heavy vertical lines indicate portions of the cores examined.
| Size group, microns | Water content, volume per cent |
|---|---|
| 250–500 | 45.0 |
| 125–250 | 45.4 |
| 64–125 | 46.9 |
| 16- 64 | 51.6 |
| 4- 16 | 66.2 |
| 1- 4 | 85.8 |
| <1 | 98.2 |
The permeability of a sediment, which is a measure of the ability of water to circulate through it, is related to the porosity. Although of undoubted importance in determining the chemical conditions and hence
Many chemical and biological processes are dependent upon the water content and the permeability, that is, upon the amount of interstitial water present and upon the rate at which water circulates through the sediment and exchanges with the overlying water. Where very little exchange with the overlying water takes place, depletion of the oxygen and the establishment of reducing conditions may occur. These conditions are inimical to the persistence of animal life, hence below the superficial layers there may be virtual exclusion of all living organisms except bacteria. The thickness of the superficial layer within which there is some exchange of water will depend upon the texture of the sediment, the rate of sedimentation, water movements, the oxygen content of the overlying water, and the activities of burrowing organisms.
The salinity of the interstitial water, which is determined by that of the overlying water, is probably not very important, although it may influence such processes as the solution or precipitation of calcium carbonate. Changes in the chemical composition of the dissolved materials are chiefly related to biological processes, although base-exchange with clay minerals, the formation of authigenic minerals, and the adsorption of ions on colloidal particles may play some part. Organic activity will lead to the consumption of dissolved oxygen and the formation of carbon dioxide which will tend to lower the pH of the interstitial water and favor the solution of calcium carbonate. If the oxygen is completely removed, anaerobic decomposition will lead to the formation of hydrogen sulphide from organic matter. Marine anaerobic bacteria are also capable of attacking the sulphate present in the water and of using the oxygen in the sulphate ion in their metabolic processes. This process also leads to the formation of hydrogen sulphide. The extent to which organic and inorganic processes within marine sediments may modify the composition of the interstitial water is not yet established, although scattered evidence indicates that they may be quite appreciable. The destruction of organic matter on the sea bottom also involves the nitrogen, and it is possible that a considerable amount of inorganic forms of this element are produced on the sea bottom. Under reducing conditions this must be ammonia, but within the superficial layers the ammonia may be further oxidized to nitrite or nitrate.
Many general statements have been made concerning the depletion of dissolved oxygen and the establishment of reducing conditions in marine sediments, but relatively little is known concerning the oxidation-reduction potentials prevailing in marine deposits (ZoBell, 1939). Although the state of oxidation of the iron and such other elements as manganese, sulphur, and nitrogen may be determined by the prevailing potential, it should be remembered that the reducing conditions are
In the overlying water in the presence of free oxygen, Eh potentials (p. 211) slightly higher than 0.0 volt are found (ZoBell, 1939). The Eh of sediments of the California coast has been found to range in general between —0.12 and —0.58 volt, but in certain areas of slow deposition slightly positive values may prevail. In general, the oxidation-reduction potential decreases (the negative values increase) with increasing depth in the sediment. The zone of most rapid change is within the topmost few centimeters. According to Hewitt (1937), aerobes tolerate an Eh between +0.4 and —0.2 volt; therefore the conditions beneath the uppermost few centimeters are not favorable for aerobic forms. The presence of “aerobes” at lower potentials indicates that they are either facultative anaerobes or that they are in a more or less dormant condition. The rapid decrease in numbers with depth in the cores is shown in table 113. Anaerobes are therefore responsible for the extremely low potentials found in marine sediments. These potentials sometimes exceed that of the hydrogen electrode, which, in neutral solution (pH = 7), has an Eh —0.421 volt. It will be seen from table 113 that the anaerobes also decrease in number with depth in the core, although the relative change is not as great as for the aerobes.
| Depth of strata (cm) | Anaerobes per gram | Aerobes per gram | Ratio of anaerobes to aerobes | Oxygen absorbed (mg/g) | Oxidation-reduction potential, Eh, volts |
|---|---|---|---|---|---|
| 0- 3 | 1,160,000 | 74,000,000 | 1:64 | 2.8 | -0.12 |
| 4- 6 | 14,000 | 314,000 | 1:21 | 1.3 | -0.29 |
| 14–16 | 8,900 | 56,000 | 1:6 | 0.6 | -0.37 |
| 24–26 | 3,100 | 10,400 | 1:3 | 0.7 | -0.32 |
| 44–46 | 5,700 | 28,100 | 1:5 | 0.3 | -0.37 |
| 66–68 | 2,300 | 4,200 | 1:2 | 0.4 | -0.34 |
Although the Eh decreases with depth in the sediment, the reducing capacity usually decreases. The reducing capacity can be determined on freshly collected samples by measuring the oxygen absorbed after the addition of mercuric chloride to kill living organisms and to inactivate
Benthic organisms, with or without skeletal structures, are involved in a number of sedimentation processes. Besides destroying organic matter, the larger benthic forms may ingest the sediment, causing mechanical abrasion of the solid particles and acceleration of the solution of such material as calcium carbonate. The turning over of the superficial layers by mud-eating and burrowing organisms tends to destroy laminations and to aid in the interchange of the water in the sediment with the overlying water. Characteristic constituents of many terrigenous deposits are faecal pellets of benthic animals (Moore, 1939). Faecal pellets have been considered as important in the formation of glauconite (Takahashi, 1939). The characteristic distribution of different forms of benthic organisms has received considerable attention both from marine biologists and from paleontologists, and their findings cannot be discussed here. In the sections on marine biology many references to this problem will be found.
CALCIUM CARBONATE
Factors Which Determine Accumulation and Deposition of Calcareous Material
Interest in geological problems concerning the conditions which led to the formation of limestones has greatly stimulated the study of the calcium carbonate content of marine deposits, because most calcareous formations were recognized as having a marine origin although in certain cases no fossil remains of marine organisms could be identified. Questions therefore arose regarding the environmental factors which determine the carbonate content of sediments laid down in different regions and regarding the possibility of inorganic precipitation of calcium carbonate in the sea. As the calcareous material in marine deposits is predominantly composed of skeletal structures of plants and animals, the marine biologist is directly concerned with the problem of the formation of such sediments. The precipitated carbonates are formed from materials in
The terms “calcareous” and “calcium carbonate” are not strictly synonymous. According to analyses (p. 231 and p. 991), calcareous skeletal structures contain variable proportions of other substances, such as magnesium carbonate. However, the percentage of materials other than calcium carbonate is generally small and for this reason is commonly neglected. Most of the calcium carbonate found in marine deposits is in the crystalline form known as calcite, whereas the calcareous structures of live organisms apparently contain at least some in the form of aragonite. Whether or not there is a selective solution of the aragonite or whether there is a transformation of aragonite to calcite after the death of the organisms is not known. As was pointed out in the discussion of globigerina ooze, much of the fine material is composed of minute calcite crystals which apparently arise from the mechanical disintegration of the foraminiferal skeletons and are not due to any process of reprecipitation. Finely divided calcium carbonate is found on certain shallow banks in low latitudes, such as the Bahama Banks. This is in the form of aragonite and is thought to have been precipitated directly from sea water without the immediate participation of organic agencies (Vaughan, 1924, Smith, 1940). The presence of small amounts of authigenic dolomite, that is, the form of carbonate containing equal proportions of calcium and magnesium, has been reported by Correns (1937, 1939) for the pelagic deposits of the Equatorial Atlantic. No satisfactory explanation has yet been advanced to account for the existence of calcium carbonate in the form of aragonite under one set of conditions and as calcite under another.
The precipitation or solution of calcium carbonate is controlled by the degree of saturation of the water under a given set of conditions. Although the conditions which favor either precipitation or solution of calcium carbonate are known, it is impossible to state definitely for a given set of conditions whether the water is saturated with calcium carbonate or whether precipitation or solution may take place. The supply of calcium carbonate by rivers is relatively large and therefore it is reasonable to assume that conditions closely approaching saturation must prevail.
In most discussions of calcareous sediments the emphasis is placed upon the percentage of calcium carbonate rather than upon the character
In chapter VI (p. 207) it was shown that conditions favorable for the precipitation of calcium carbonate are most likely to occur in water having high temperature and high salinity and where the activity of plants has reduced the carbon dioxide content of the water. Such conditions are found in low latitudes over shallow bottoms, but apparently in a few localities only does such precipitation occur. Smith (1940) has described the precipitation of aragonite that occurs on the Bahama Banks (see also Vaughan, 1924) and has pointed out that there the process is facilitated by seeding with the minute aragonite crystals stirred up from the bottom.
Precipitation through organic agencies does not require that the sea water be saturated with calcium carbonate. However, deposits high in calcareous skeletal structures are generally restricted to areas where the physical-chemical environment is such that the ionic product [Ca++] X [CO3−] is large (see p. 205). The photosynthetic activity of plants, which removes carbon dioxide from solution and hence tends to increase the concentration of CO3−ions, offers a simple mechanism for
Transportation is of less importance to the distribution of calcareous material than to that of certain other constituents of the sediments. The benthic forms are large and can be moved only by the action of waves or extremely strong currents. However, wave action, boring organisms, and organisms which feed on the bottom mud tend to break down the larger fragments and, in this way, coral sands and muds are formed. Such a breakdown may favor a certain amount of solution. Agencies of lateral transportation are not very important even for those pelagic forms which live in the upper layers, since their skeletons sink quite rapidly and are not transported far from the region of development.
The potential supply of calcareous material to the bottom is dependent upon the production of calcareous forms either in the plankton of the overlying water or among the benthic organisms. However, a certain amount of solution may take place after the death of the organism and may occur either while settling through the water or on the sea bottom. Whether or not solution will take place depends upon the physical-chemical conditions prevailing in the water but, as the exact value for the solubility product is as yet unknown, it is impossible to state whether or not the waters of the oceans are saturated with calcium carbonate. Earlier studies lay particular emphasis upon the solution which takes place while the calcareous material is settling through the water. Examinations of the vertical distribution of calcium and alkalinity in the Pacific and Atlantic Oceans show that usually the lowest values are found in the surface layer where a certain amount of precipitation, probably through organic agencies, has taken place. Within this surface layer the concentrations are approximately the same in both the Atlantic and the Pacific Oceans, but at greater depths the existing data indicate that the waters of the Pacific contain somewhat more calcium and have a higher alkalinity: chlorosity ratio than those of the Atlantic. The larger values in the Pacific can probably be correlated with the lower oxygen content of the intermediate and deep water. The consumption of oxygen at subsurface levels is accompanied by an increase in the total carbon dioxide and therefore tends to favor the solution of any calcareous material which may settle through the water. However, it should be remembered that the consumption of oxygen, hence the production of carbon dioxide in deeper waters, is independent of the supply of calcium carbonate settling from above. Therefore we may expect that in certain localities the subsurface waters are undersaturated, whereas in other areas where there has been an adequate supply of calcareous material the deeper waters may be completely saturated with calcium carbonate.
Calcareous material on the sea bottom is exposed to the water, and if the water is undersaturated must pass into solution. In the presence of calcareous material there must be a zone within which the water is saturated with calcium carbonate and additional solution can take place only when this water is replaced by currents or by eddy diffusion. The rate at which calcium carbonate can be dissolved therefore depends upon the character of the flow and the gradients which are established and which for a given set of conditions cannot exceed a certain upper limit. If the rate of supply of calcium carbonate is greater than the rate at which the dissolved material can be carried away there will be a net gain in the amount of calcareous material and, consequently, accumulation will take place. If the supply is less than the rate at which it can be dissolved there will be no accumulation of calcareous material although considerable amounts may fall to the sea bottom in that locality. Besides the solution which may take place if the overlying waters are undersaturated, the production of carbon dioxide on the sea bottom, a result of the decomposition of organic matter, will favor the solution of calcareous material. Even if the overlying water is saturated with calcium carbonate this process will lead to a certain amount of solution. The number of benthic organisms is largely determined by the supply of decomposable organic matter and, in addition to the production of carbon dioxide, their activities in burrowing and as mud eaters will favor the breakdown of calcareous material. Extensive observations by Wattenberg in the Atlantic Ocean show a characteristic increase in the alkalinity: chlorosity ratio immediately over the bottom, which indicates a certain amount of solution of calcareous material. Furthermore, Wattenberg's data show a decrease in the dissolved oxygen content immediately adjacent to the bottom, indicating active production of carbon dioxide.
Calcium carbonate is most likely to dissolve in water which has come from high latitudes and in which the carbon dioxide content has been increased by oxidative processes. Water from high latitudes fills the great ocean basins below depths of a thousand meters or more. In some parts of the South Atlantic Ocean there is a fairly well-defined northward flow of water immediately over the bottom and, according to the results of the Meteor expedition, such areas are poor in calcium carbonate. As the water moves toward the Equator it undoubtedly takes up more calcium carbonate and can therefore dissolve less and less, for which reason alone we may expect to find calcareous sediments in low latitudes. In areas of large organic production where there is an abundant supply of organic matter to the sea floor, as along coasts where there is upwelling, and in areas of diverging currents and active winter mixing in high latitudes, we may expect to find sediments rather low in calcium carbonate because of the large amount of solution which can take place on the bottom.
The Distribution of Calcium Carbonate
The distribution of calcium carbonate in pelagic deposits has already been discussed in some detail. In fig. 258 the percentage of calcium carbonate in the superficial layers of the sediments is shown without regard to the type of deposit or to the depth. This map, which is based on that of Trask (1937), shows only the 10 per cent and 50 per cent contours. However, as pointed out before, the transition from sediments low in carbonate to those high in calcareous material usually occurs in a relatively short distance. The 30 per cent contour which limits the pelagic calcareous deposits from the red clay and siliceous types is shown in fig. 253. Trask's map has been revised to take into account more recent data, from Schott (1939a) for the Indian Ocean, Revelle (1936) for the Pacific Ocean, and Correns et al (1937) and Pratje (1939) for the Atlantic Ocean. Figure 258 shows that sediments containing more than 50 per cent calcium carbonate are restricted to latitudes lower than 50 degrees except in the North Atlantic, where calcareous sediments extend considerably further north. A comparison of figs. 253 and 258 shows that there is no abrupt change in calcium carbonate content with distance from shore that would correspond to the transition from terrigenous to pelagic deposits. The sediments near shore and in enclosed areas are by definition described as “terrigenous,” but as can be seen from fig. 258 no such demarcation exists in the distribution of calcium carbonate. Off most continental coasts within low latitudes there is an increase in calcium carbonate content with increasing distance from the coast. This is particularly marked in regions where there is a transport of cold water from high latitudes and upwelling, as off the west coast of South America. Furthermore, in regions where there is an abundant supply of inorganic material as along coasts of heavy rainfall and off the mouths of rivers, the carbonate content is generally low. One of the striking features of the carbonate distribution is the very low values found in the North Pacific. A possible explanation for this anomalous distribution will be discussed below. It will also be noted that the carbonate content of the deeper parts of the ocean basins is generally low.
Trask (1937) has studied the distribution of the percentage of calcium carbonate in marine sediments to determine the relationships between surface temperature, surface salinity, depth, distance from shore, and the calcium carbonate content of the underlying sediments. This statistical study showed a positive correlation between the carbonate content and the salinity of the overlying surface water. When salinities were less than 34 ‰ the carbonate content was generally less than 5 per cent, whereas in regions where the surface salinity exceeded 36 ‰ the calcium carbonate was generally greater than 50 per cent. The calcium carbonate content also increased with increasing surface temperature but decreased with depth in deeper water. In general, nearshore sediments have considerably smaller contents of calcium carbonate than the pelagic deposits although this relation will not necessarily hold in low latitudes. The correlation obtained by Trask between the carbonate content of the sediments and certain conditions in the surface layers indicates the importance of the potential supply of calcareous material. Surface conditions are, in general, no indication of those which prevail at lower levels and which would determine whether or not the calcareous material would redissolve while sinking or after reaching the bottom. That is, the agreement which Trask obtained between the carbonate content of the sediments and the temperature and salinity conditions in the surface waters merely emphasizes the fact that calcareous deposits will accumulate in areas where the conditions favor the development of carbonate-secreting organisms.
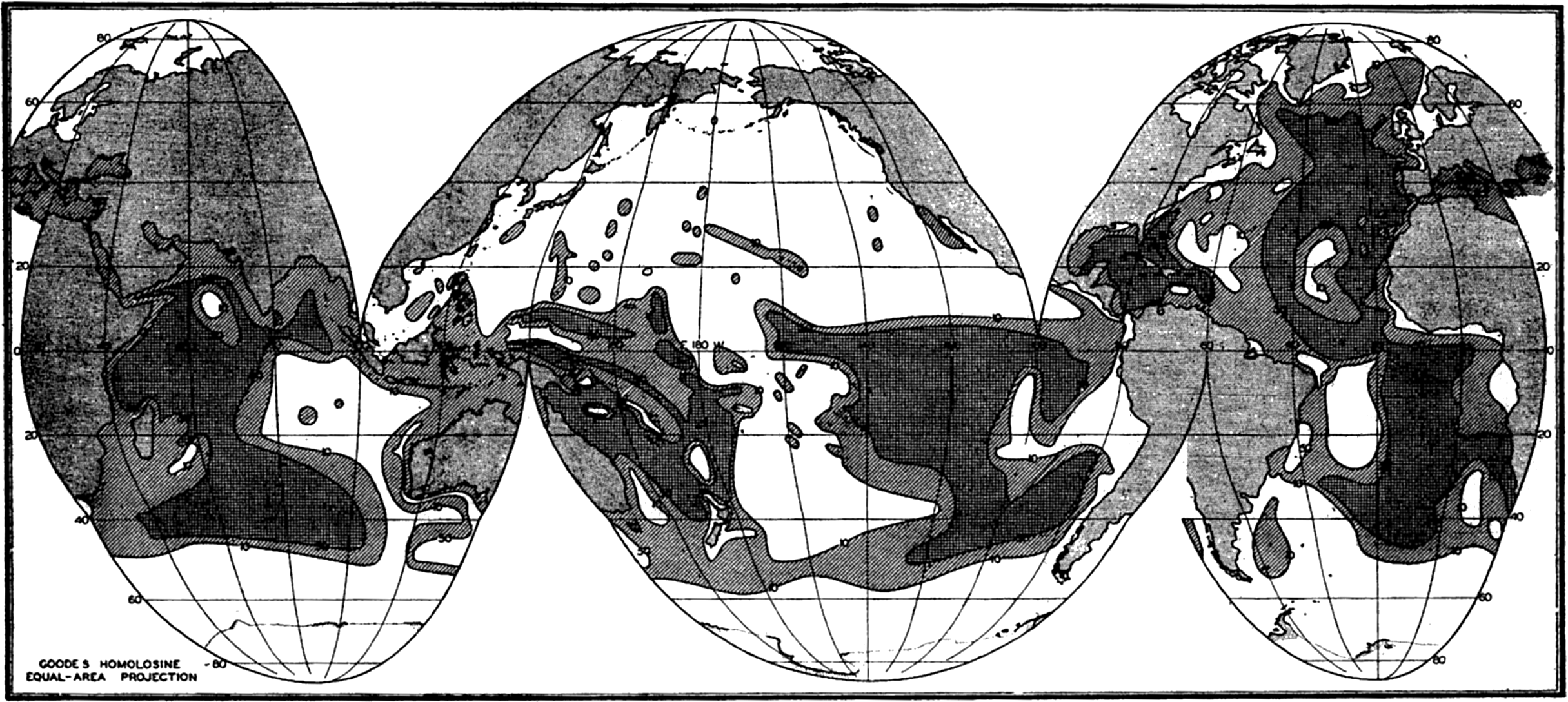
Percentage of calcium carbonate in marine sediments. (Modified from Trask, 1937.)
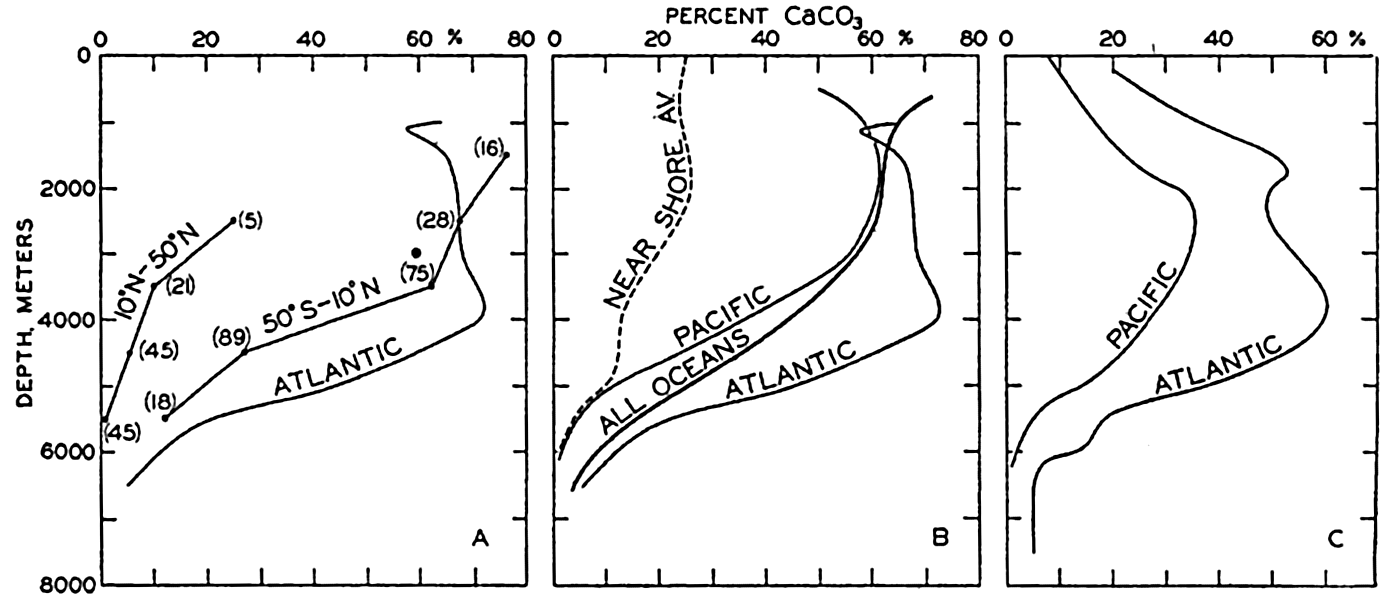
Percentage of calcium carbonate in marine sediments as a function of depth. (A) Averages for pelagic sediments from the North Pacific (10°N to 50°N), South Pacific (50°S–10°N), and from the Atlantic Ocean. (B) Averages for pelagic sediments from the Atlantic and Pacific Oceans and from all oceans, and the average curve for nearshore deposits. (C) Averages for all types of sediments from the Atlantic and the Pacific Ocean.
Revelle (1936) examined the distribution of calcium carbonate in the sediments of the Pacific Ocean with reference to the variations with latitude and depth. The average values arranged according to latitude and depth for the pelagic deposits are shown in table 114. The decrease in carbonate with increasing latitude, that is, decreasing surface temperatures, and the small percentages found at greater depths are readily seen. It will also be noted that the calcium carbonate content of sediments from the North Pacific is less than that of material from the corresponding range in latitude and depth from the South Pacific. The marked decrease does not take place exactly at the Equator, but apparently at about 10°N. The curves obtained when these data are averaged in two groups, one for the North Pacific between 10°N and 50°N, and
| Depth range (meters) | Latitude | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50°–40°S | 40°–30°S | 30°–20°S | 20°–10°S | 10°S–0 | 0–10°N | 10°–20°N | 20°–30°N | 30°–40°N | 40°–50°N | |
| 0–1000 | 41.0 | |||||||||
| 1000–2000 | 94.0 | 74.4 | 75.0 | 38.4 | ||||||
| 2000–3000 | 82.0 | 74.7 | 63.5 | 65.9 | 68.0 | 57.8 | 32.5 | 18.0 | 22.0 | |
| 3000–4000 | 63.6 | 53.4 | 72.3 | 54.0 | 49.4 | 0.6 | 11.7 | 56.0 | 6.0 | |
| 4000–5000 | 37.5 | 18.8 | 17.9 | 19.9 | 47.5 | 55.9 | 17.6 | 1.9 | 1.8 | |
| >5000 | 0.0 | 16.0 | 10.7 | 24.7 | 1.0 | 0.8 | 0.6 | 0.5 | ||
The data in fig. 259A are presented to emphasize the differences between the average calcium carbonate content as a function of depth in the North and South Pacific and in the Atlantic Ocean. In fig. 259B the curve for the pelagic deposits of the Atlantic Ocean is repeated and the average for the Pacific Ocean as a whole is shown. Also included in this diagram are Trask's data for pelagic deposits which represent an average for all oceans. In addition, a curve based on Trask's average for nearshore sediments is included to show the lower calcareous content of terrigenous deposits. It is immediately noticed that the Atlantic Ocean is high in percentage of calcium carbonate compared to the Pacific. In fig. 259C are shown the vertical distribution of the percentage calcium carbonate where all samples, both terrigenous and pelagic, are combined. The data for the Pacific are from Revelle (1936) and those for the Atlantic are from Pia (1933). Unfortunately, Trask's data have not been combined to give a grand average for both nearshore and pelagic sediments. The differences between nearshore and pelagic
The extreme and the average carbonate contents of the various types of marine sediments have been given in table 108. Vaughan (1924) calculated the average calcium carbonate content of marine sediments from the Challenger values for the different types of deposits and the area covered by each type. In this way the following values were obtained:
| Average, all deposits | 32.2% CaCO3 |
| Average, pelagic deposits | 33.4% CaCO3 |
| Average, terrigenous deposits | 24.6% CaCO3 |
More complete data concerning the calcium carbonate content and the areal extent of the different types of deposits would modify these values somewhat. The data presented in fig. 259 can be used to estimate the average calcium carbonate content when the area represented by the different depth intervals is known (table 5, p. 21). Trask's figures for the calcium carbonate content of the pelagic deposits when treated in this way give an average of 37 per cent CaCO3. The data for the Pacific and the Atlantic (fig. 259B) give averages of 27 per cent and 54 per cent, respectively. When all types of sediments are combined (fig. 259C), we obtain an average of 18.8 per cent CaCO3 for the Pacific Ocean and 41 per cent for the Atlantic Ocean. The available data therefore indicate that the average calcium carbonate content of pelagic deposits is about 37 per cent and for terrigenous sediments about 25 per cent. Owing to the greater frequency of sampling in intermediate and low latitudes, the values for the terrigenous sediments may be somewhat high. The data in Clarke (1924, p. 34) indicate that the mean value for fossil-bearing shallow-water deposits is probably about one half of this, namely 12.5 per cent.
It should be pointed out that the averages given above are those for a superficial layer of the marine deposits and represent the averages for a certain thickness, regardless of the rate of deposition. The average obtained in this way, therefore, does not correspond to the ratio between the calcareous and noncalcareous material which may be carried to the sea. In order to obtain a balance sheet for the supply and deposition it is necessary to take into account the relationship between the rate of deposition and the calcium carbonate content. As shown on p. 1036, the terrigenous sediments accumulate much more rapidly than the pelagic deposits and, because of their lower calcium carbonate content, the ratio of the rates of accumulation of calcareous and non-calcareous material is 1:5.2, that is, the percentage of calcium carbonate in the total annual sedimentation is 19 per cent.
It is impossible to discuss the pattern of distribution of percentage of calcium carbonate in detail, and only a few of the major features can be considered. These are (1) the higher calcium carbonate content of the sediments of intermediate and low latitudes and the somewhat anomalous northward extension of calcareous sediments in the North Atlantic, (2) the decrease in calcium carbonate content with increasing depth in pelagic deposits, (3) the possible reasons for the difference between the carbonate content of the sediments in the Atlantic and the Pacific Oceans and between those in the North and South Pacific. Owing to the incomplete understanding of the processes involved, only the general character of the controlling agencies can be pointed out.
The contrast between the calcium carbonate content of the sediments in intermediate and low latitudes on one hand and in high latitudes on the other is undoubtedly related to the higher production of calcareous forms in low latitudes. In fact, there are virtually no calcareous planktonic forms found in high latitudes. Another factor which may contribute to the difference is that of solution of the calcium carbonate either in the water or after it has reached the bottom. As shown elsewhere, conditions favorable for the solution of calcium carbonate are characteristic of high latitudes in contrast to those in low latitudes. The relative rate of accumulation of noncalcareous material may play some part in determining the general distribution, but it is considered as secondary compared with the absolute rate of supply of calcareous material.
The decrease in the percentage of calcium carbonate with depth in pelagic deposits may be attributed to a number of factors. Possibly the most important is the effect which topography will have upon the deposition of extremely fine-grained inorganic debris. Such debris will tend to accumulate in depressions where it will be deposited more rapidly than upon topographic highs, and hence will tend to dilute the calcium carbonate. Two other factors must be considered. The first of these, namely the amount of solution during sinking, has been considered to be an important factor since the material which is deposited at great depths has to pass through a longer column of water and is therefore exposed to the solvent action for a longer period. The second factor is that of solution on the bottom. If there is a greater supply of decomposable organic material to the deep basins, the larger production of carbon dioxide may tend to dissolve more of the calcium carbonate which reaches the bottom. All of these factors may be effective in determining the decrease in the percentage of calcium carbonate with increasing depth in the open ocean.
In order to present any explanation for the difference in character between the sediments in the Atlantic and Pacific Oceans and the sediments of the North and South Pacific, it is helpful to return to the concept of the stationary distribution of properties in the water. As
It is more difficult to offer any definite reason for the difference between the North and the South Pacific. Most of the supply by rivers is actually in the North Pacific, therefore there must be a net transport of calcium across the Equator. The lower calcium carbonate content of the sediments may be due to the greater relative rate of accumulation of noncalcareous material or to the slower relative rate of deposition of calcareous material. Conditions within the water itself may also play a part. The deep-water circulation of the Pacific is such that the water flowing in from the south must ultimately turn around and flow south again (p. 752). There is a general decrease in the oxygen content and, hence, an increase in the carbon dioxide content from south to north. Therefore, conditions in the North Pacific may be more favorable for the solution of calcium carbonate both in the water and on the bottom. Further studies of the chemistry of the waters and more adequate knowledge of the solubility of calcium carbonate will make possible a more exact analysis of these various factors.
ORGANIC MATTER
Quantity and Character of Organic Matter in Marine Sediments
The term organic matter is used to designate that portion of a sediment which has arisen through organic activity and which contains carbon in any form other than carbonate. Because it is somewhat analogous to the humus fraction found in soils it may be termed “marine humus.” Sometimes the expression decomposable organic matter is used to indicate that the material may be destroyed by organisms, particularly by bacteria.
The quantity and character of organic matter in marine sediments has been studied by a number of workers whose findings have been reviewed by Trask (1932, 1939). Although the organic matter rarely forms more than a small percentage of the sediments, it has received considerable attention for the following reasons:
The organic matter settling to the sea bottom in deep water is the only source of food for benthic animals and bacteria. Hence the quantity in the sediment and, particularly, the rate of supply in any locality will have a profound effect upon the population of bottom-living organisms.
The presence of organic matter and the changes brought about by organic activity, principally bacterial, influence the physical and chemical conditions which prevail in the sediment. These in turn may affect the character of the bottom fauna and the nature of the deposit.
The burial of the organic matter implies the removal from the water of carbon, nitrogen, and possibly other elements originally in solution.
Organic matter in sedimentary formations is considered to have been the source of petroleum. This theory has stimulated most of the investigations on the organic-matter content of recent sediments, as it has been thought that they would yield results which could be applied to the study of the source beds of petroleum and in the search for new oil fields.
The organic-matter content of pelagic sediments is approximately 1 per cent. Nearshore sediments generally contain somewhat larger amounts and average about 2.5 per cent. The extreme range is from less than 0.5 to more than 10 per cent. According to Trask (1939) the sediments of the North Pacific contain from 1 to 1.5 per cent, those of the South Pacific from 0.4 to 1.0 per cent, and those of the Atlantic from 0.3 to 1.5 per cent. The high values are found in bays and estuaries, in isolated basins near shore, in fjords, and in such landlocked regions as the Black Sea, Trask (1932) summarized the data available at that time concerning the distribution of organic matter in different areas and in different types of environments. Since then Gripenberg (1934) has investigated the Baltic Sea, Ström (1936) has made detailed studies of the Norwegian fjords, and Revelle and Shepard (1939) have reported on the conditions off the coast of California. The Meteor samples from the equatorial Atlantic have been discussed by Correns (1937, 1939) and the Carnegie samples of the Pacific were studied by Revelle (1936). Wiseman and Bennett (1940) have reported on the conditions in the Arabian Sea. Trask (1939) has again summarized the existing knowledge and given many references to detailed studies.
The quantity of organic matter may be estimated in various ways. The determination of the noncalcareous carbon by either wet or dry
Little is known concerning the chemical composition of the organic matter in marine sediments. Carbon forms from 50 to 60 per cent of the material, hence the ratio of organic matter to carbon varies between 1.67 and 2.0. Trask recommends the ratio of 1.8, which corresponds to a carbon content of 56 per cent. Various factors have been shown to influence the ratio. Plant material has a somewhat higher ratio than organic matter of animal origin and under stagnant conditions the ratio is lower than average. The nitrogen content of the organic matter is also affected somewhat by the source of the material and the conditions prevailing in the sediment. The carbon: nitrogen ratio varies between 8 and 12 and averages about 10, with the extreme values found by Trask as 5.5 and 20. Wiseman and Bennett (1940) found an even greater spread in the ratios, and for this reason do not consider nitrogen as an adequate measure of the organic matter. The average recommended by Trask is 10, which implies that the organic matter contains 56 per cent carbon and 5.6 per cent nitrogen. The organic matter which accumulates on the sea bottom represents the more or less resistant fraction of plant and animal origin. Although no specific compounds have been isolated it is possible to obtain some idea as to the character of the compounds by making selective extractions and analyses. The protein content is estimated by multiplying the organic nitrogen by the conventional factor of 6.25. Extraction with a fat solvent, such as ether or carbon tetrachloride, is made to determine the fatty materials present. By subtracting the protein and ether-soluble material from the total amount of organic matter the carbohydrate fraction is determined. Tables 115 and 116 (from Trask, 1939) show the character of the organic matter in various types of organisms and in marine sediments.
| Organic-matter source | Crude protein | Ether extract | Carbohydrates | Crude fiber |
|---|---|---|---|---|
| Peridineans | 14 | 1.5 | 85? | ? |
| Diatoms | 29 | 8 | 63 | 0 |
| Copepods | 65 | 8 | 22 | 0 |
| Higher invertebrates | 70 | 10 | 20 | 0 |
| Organic matter in marine sediments | 40 | 1 | 47 | 0 |
The ultimate source of the organic matter is within the euphotic zone, either in the sea or on land. The original plant material may find its way to the sea bottom or it may have undergone transformation into animal remains or their waste products or it may have been converted into bacterial cell structure before it is deposited. Organic matter produced on land may play a relatively important role in bays and estuaries and in areas of excessive runoff and low plankton production, such as the Baltic Sea (Gripenberg, 1934). Rafts of land vegetation may be carried away from the coast and fragments of these may settle to the sea bottom. In shallow water, particularly in relativley isolated environments, such attached aquatic plants as eel grasses have been shown to be important sources of organic matter (p. 303). However, for the ocean as a whole, the supply of organic material from land and from the attached plants is relatively insignificant and of local importance only, and it is the planktonic photosynthetic organisms which are the chief source of organic matter for marine sediments.
| Organic-matter source | Percentage by weight | Ratio of organic matter to carbon | |||
|---|---|---|---|---|---|
| Carbon | Nitrogen | Hydrogen | Oxygen | ||
| Peridineans | 45 | 3 | 7 | 45 | 2.2 |
| Diatoms | 50 | 6 | 8 | 36 | 2.0 |
| Copepods | 50 | 10 | 8 | 32 | 2.0 |
| Marine sediments | 56 | 6 | 8 | 30 | 1.8 |
| Lithified sediments | 64 | 4 | 9 | 23 | 1.6 |
Since the potential supply to the bottom depends upon the production within the upper layers, there must be regional differences in the amount of organic matter in sediments related to the variations in the production. The organic material is light and sinks slowly, and it behaves in a manner similar to the finer fractions of the solid material. This resemblance is shown by the inverse relationship between the organic-matter content and the median diameter of the sediments in nearshore areas (Trask, 1932, 1939, Revelle and Shepard, 1939).
The cycle of regeneration of the elements involved in organic processes, that is, of decomposition of organic matter, has been described. It was shown that regeneration is relatively complete and that probably the greater part of the organic matter produced by the plants is mineralized before reaching the sea bottom. The amount of decomposition that will take place within the water depends upon a number of factors, such as the relation between plant production and consumption by the animals, the number and type of bacteria, and such other characteristics as the temperature of the water. The destruction of organic matter is a time-consuming process and will depend not only upon the organic activity but also upon the period during which such action can take place. For this reason the depth of water through which the organic matter may sink must be considered. The deeper the water, the longer the interval required to reach the bottom and the more likely the organic matter is to be destroyed. The presence or absence of dissolved oxygen in the water may have a profound effect upon the fate of the organic matter produced in the surface layers. In stagnant waters the destruction of organic material is not as complete as under oxidizing conditions and, hence, will tend to favor the deposition of organic matter on the sea floor. The factors that contribute to the development of stagnation are discussed elsewhere (p. 1026). One of these is the abudnant supply of organic material to the sea bottom in the absence of an adequate supply of oxygen; hence an abundant supply of organic matter tends to favor its own preservation.
Thus far, we have considered only the agencies operating within the water column. After reaching the sea bottom and even after burial, the transformation and destruction of organic matter by the bottom fauna and microorganisms is continued. The character and extent of these changes is again largely dependent upon the presence or absence of dissolved oxygen. The amount of organic matter reaching the bottom will tend to determine the number of benthic animals and microorganisms. In the absence of free oxygen only anaerobic organisms can thrive, hence the processes operative will be of a character dependent on this situation.
The relative rate of deposition of organic matter when compared with the nondecomposable material contributed to the sediment is an important factor. Although the supply of organic matter may be identical in two areas, the concentration and character of the material in the sediments may be profoundly different if there are different rates of deposition of the nondecomposable material. The latter will tend to dilute the organic matter and, furthermore, in areas of rapid deposition, burial soon after reaching the sea floor reduces the destruction by benthic animals. As emphasized elsewhere, the physical-chemical environment immediately at the surface of the sediment may be quite different from that only a few centimeters beneath. Where deposition is relatively rapid and,
From the foregoing discussion it is obvious that the environment of deposition, that is, the biological and physical-chemical conditions on and in the sediment, are probably the most important factors in determining the distribution of the organic matter. If conditions are established which are favorable to the preservation of organic matter, these will tend to be maintained.
To summarize the above generalizations, the following conditions will favor the formation of sediments rich in organic matter:
An abundant supply of organic matter.
A relatively rapid rate of accumulation of inorganic material, particulary if fine-grained.
A small supply of oxygen to the waters in contact with the sediments. The most extreme development of such conditions may be found in basins and landlocked regions where stagnation exists with the resulting exclusion of animal life.
The following factors will tend to favor the destruction of the organic matter and the formation of sediments low in organic material:
A small supply of organic matter.
Relatively slow rate of accumulation of nondecomposable material.
An abundant supply of oxygen.
With the above-mentioned points in mind, some of the differences in distribution of organic matter in oceanic sediments can be explained. The slow deposition under oxidizing conditions, characteristic of the pelagic sediments, leads to a relatively complete destruction of the organic matter, whereas in nearshore areas where deposition is rapid and in basins where stagnant conditions prevail, a much larger percentage of the organic matter may escape destruction. Furthermore, the absolute supply is probably smaller in oceanic areas than in the coastal regions where the organic production is generally greater.
Distribution of Organic Matter
Within restricted areas where the supply of organic matter to the bottom may be considered constant, it is possible to attribute local irregularities in the organic-matter content of the sediments to the effects of transporting agencies and topography. For example, in the southern California area (fig. 260) the organic content is low on the ridges and banks and high in the depressions and basins. These differences may be attributed to the more active benthic fauna that inhabit the topographic highs and the effects of currents which sweep the finely divided organic matter and the fine-grained sediments into the depressions. The latter environment favors the preservation of the organic matter.
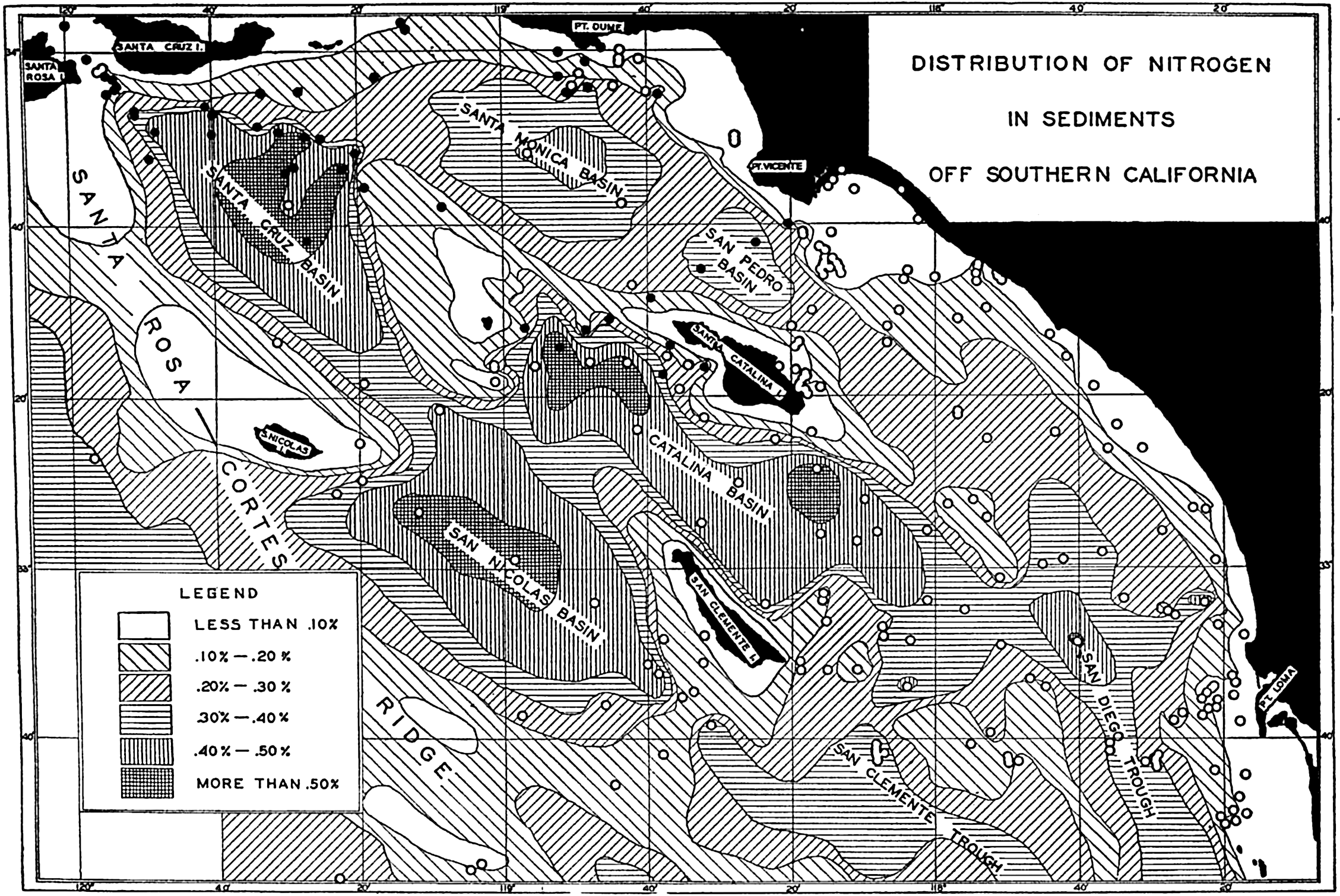
The distribution of organic nitrogen in the sediments off the southern California coast. Compare with figs. 7 and 264. (From Revelle and Shepard, 1939, in Recent Marine Sediments, edited by Parker D. Trask. American Association of Petroleum Geologists, Tulsa.)
The organic-matter content of terrigenous deposits is on the average higher than that of the pelagic deposits. According to Trask (1939), the averages are 2.5 and 1.0 per cent, respectively. As shown elsewhere (p. 1036), the rate of deposition of pelagic sediments is much less than that of terrigenous sediments, hence organic matter accumulates much more rapidly in nearshore deposits than in those of the open oceans. Trask (1939) assumed that the production of organic matter was uniform over the entire area of the oceans and attributed the more rapid accumulation in terrigenous deposits to the fact that the environment was less favorable to the destruction of organic matter on the sea bottom. However, the production cannot be assumed to be constant and the difference may be due entirely to the greater production in nearshore areas, particularly in those where upwelling and mixing are active. Until additional information is available concerning the differences in the supply of organic matter to the sea bottom it is impossible to evaluate the relative importance of supply and preservation as factors determining the organic content of the sediments. Correns (1937) attributes the higher organic content of the sediments near the African coast in the equatorial Atlantic to the larger production and, hence, greater potential supply in this region. Similarly, Wiseman and Bennett (1940) attribute the higher organic content of the nearshore sediments in the northwestern part of the Indian Ocean to the greater organic production in such areas.
Among the pelagic deposits there is less range in organic matter content. The fragmentary data available indicate that diatom oozes are the richest, followed by red clay and the calcareous oozes. Terrigenous diatomaceous deposits such as those in the Gulf of California (Revelle, personal communication) are among the richest group in their content of organic matter and may contain more than 10 per cent.
Studies of the variations in organic-matter content with depth and distance from shore generally show maxima at intermediate depths and distances. In some cases this feature is associated with basin conditions, as in the southern California area; in others it may be ascribed to the greater production or more rapid accumulation of clastic material or a combination of such factors near shore. The coarse sediments of the beach and shelf are generally low in organic matter.
Trask (1939) has summarized the organic content found in various types of shallow-water environments. These data show that shelf sediments generally contain between 2.0 and 3.0 per cent with higher values
Within the marine sediments there is generally a decrease in organic content with depth in the deposit. Correns (1937), Revelle and Shepard (1939), and Wiseman and Bennett (1940) have all reported this characteristic distribution. Within the upper 50 cm of the core as collected, the organic matter generally decreases to about two thirds of its value in the superficial layers. Irregularities in the content may usually be correlated with changes in the character of the deposit. The decrease is attributed to destruction due to bacterial activity after burial. The decrease in organic matter with depth in the sediment is accompanied by a marked decrease in the number of bacteria. Offhand, it might appear that the residual organic matter is of such a type that it cannot be attacked by bacteria. However, this is certainly not true of terrigenous sediments, and the meager data indicate that the organic matter in pelagic deposits is also subject to bacterial destruction. When brought into the laboratory, marine sediments will support large and active bacterial populations which rapidly attack the organic matter (Anderson, 1939). Under the environment prevailing in the buried sediment on the sea bottom, it is possible that biological activity has practically ceased. Nevertheless, viable bacteria are always present and if the environment is changed, say, by bringing the material into the laboratory, active growth and metabolism will result in the further alteration and oxidation of the organic matter.
The percentage of organic matter has been shown to be inversely related to the texture and it has been pointed out that the oxidation-reduction potential may be related to the organic content. Various workers have drawn attention to relationships between the organic matter and other constituents of the sediments. Correns (1937, 1939) obtained a linear relationship between organic matter and calcium carbonate and concluded that the calcareous material contained about 0.2 per cent organic matter and that the noncalcareous material contained a constant proportion of organic matter. Correns’ data also show a general relationship between the organic matter and the phosphate in the sediments. Revelle (1936), from an examination of the Carnegie
SHALLOW-WATER AND NEARSHORE SEDIMENTS
The differences between sediments found in marginal and nearshore regions and those of the open ocean can be largely attributed to the great intensity of transporting agencies in shallow water. In addition to the currents which are generally relatively strong in shallow water, the surface waves create strong disturbances and play an important role in the nearshore environment. Many of the transporting agencies effective in shallow water are subject to intermittent fluctuations that may produce stratification of the sediments. Such vertical variations in the character of the sediments as are transitory and may be destroyed by the rising tide or the next storm are lacking in deep-sea deposits, where variations in the properties of the sediments represent environmental changes with time over periods of a year to thousands of years.
Because of the stronger currents, sediments in shallow water are generally coarser and better sorted than those of the open sea. The form of the coast line and the topographic irregularities of the sea bottom have marked effects upon the supply and transportation of the sedimentary debris, leading to great local variations in the character of the sediments found in shallow water. The following discussion will apply to the sediments upon the shelf and also to those on the beach, unless specifically excepted.
Water movements associated with surface waves probably do not extend below about 100 m and it is only in very shallow water and on the beach that their motion is violent. Waves breaking on the shore are capable of transporting large amounts of sediment. During storms the coarser material is thrown high on the beach above the normal high-tide level, while at the same time the lower part of the beach is eroded and a certain amount of material carried out below low-tide level. During periods of very large waves the whole beach may undergo temporary erosion. Between storms the smaller waves return sediment from shallow water to the beach (Shepard and La Fond, 1940). Rip currents that are associated with the waves contribute to the removal of sediment from the beach (Shepard, Emery, and La Fond, 1941). In the absence of currents, waves are not effective in transporting sediment parallel to the shore unless they strike the beach obliquely or unless there is a gradient in the sediment placed in suspension by the waves.
The rise and fall of the tide once or twice each day changes the level of attack of the waves on the beach. The upper part of the beach is exposed for longer or shorter periods, depending on the elevation above mean-tide level. The beach profile and the character of the sediments are undoubtedly associated with the range in tide, but no study of this
The motion associated with semipermanent currents, internal waves, eddies, and tsunamis has been discussed elsewhere and the roles of various types of water movements in sediment transportation described (p. 959). As most currents have their greatest velocity parallel to the coast they tend to produce movement of sediment alongshore. Transportation away from shore can be produced by waves and rip currents, and can take place where there is a gradient normal to the coast in the concentration of suspended material or where the slope of the bottom is such that the gravitational component facilitates movement down the slope and away from the coast.
Relation between texture of sediments and slope of beaches on the east and west coasts of the United States. Median diameter in millimeters. (After data in U. S. Beach Erosion Board, 1933.)
Supply of Sedimentary Material. Beach and shelf deposits are predominantly of inorganic origin, and only in low latitudes along coasts of small runoff and around oceanic islands are the deposits high in organic calcareous debris. The nearshore zone is favorably located to receive terrigenous and volcanic material, both water-borne and carried by the atmosphere. In addition to these sources of terrigenous and volcanic material, sedimentary material is supplied by erosion of the exposed coast
The profile of the beach, the texture of the sediments, and hence, indirectly, the composition, are all closely related and are determined by such factors as the range in tide, exposure to waves and storms, and the character of the material forming the coast. The relationship between the slope of the beach and the median diameters of beach sediments on the east and west coasts of the United States is shown in fig. 261.
It is commonly stated that the inner part of the shelf represents a wave-cut terrace and that the outer extension is a wave-built platform composed of the materials carried out from the land and eroded from the shallower depths. Although wave-built terraces may be accumulating in certain localities, they are not a common feature of the continental shelf because (1) rock exposures are commonly found on the seaward edge of the shelf; (2) submarine canyons cutting across the shelf have steep and often rocky walls, even at the outer edge of the shelf; (3) the continental slope is commonly too irregular to have been produced by the accumulation of a large amount of unconsolidated material. As only the fine material is transported far out to sea, the bulk of the sediment carried away from continental coasts must accumulate either on the shelf or on the continental slope. Considerable evidence has been gathered which indicates that little or no deposition occurs on the shelf. In any coastal area in which this is true there can be no selective sorting of the material while in transit across the shelf. If there is a net accumulation, sorting may take place, leaving the coarser grains in the shallower or more exposed localities. In general, the bulk of the inorganic debris must accumulate in depressions on the shelf or immediately beyond the continental slope.
Beach and Shelf Sediments. Sediments of the beach and shelf show large vertical and lateral variations in texture. In terms of median diameters the range is from greater than 256 mm to less than 0.1 mm, namely, from boulders to fine silt. Numerous references to studies can be obtained in the symposium on Recent Marine Sediments (Trask, ed., 1939). Martens (1939) has summarized data on the textural characteristics of beach deposits from the eastern United States (table 117). These data show that the extreme range in median diameters is from 1.25 mm to 0.13 mm, but that the averages for different localities show a relatively small spread, namely, from 0.21 to 0.35 mm. The small coefficients of sorting emphasize the often stated fact that beach deposits are well sorted. The skewness values greater than unity show the predominance of the larger grades. Beach sediments on the California coast show
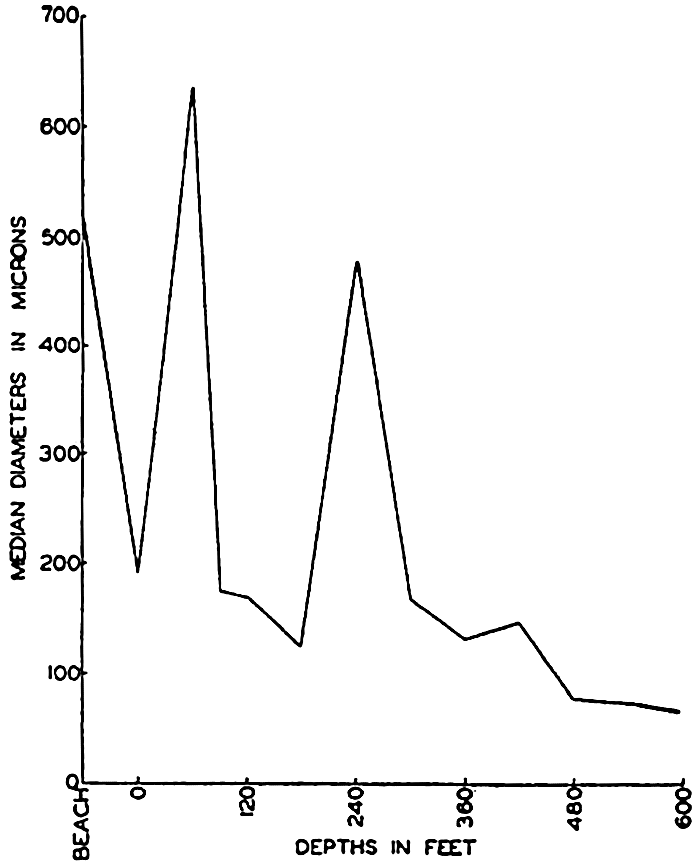
Average of median diameters of sediments from different depth zones in Santa Monica Bay, California. (From Shepard and Macdonald, 1938.)
| Region | No. of samples | Median diameter (mm) | Coefficient of sorting | Coefficient of skewness | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Max. | Min. | Average | Max. | Min. | Average | Max. | Min. | Average | ||
| South shore of Long Island | 22 | 0.48 | 0.23 | 0.35 | 1.41 | 1.16 | 1.32 | 1.15 | 0.91 | 1.01 |
| New Jersey | 37 | 0.70 | 0.15 | 0.33 | 1.53 | 1.14 | 1.27 | 1.47 | 0.88 | 1.03 |
| Del., Md., and Va. north of Chesapeake Bay | 18 | 0.54 | 0.15 | 0.30 | 1.60 | 1.13 | 1.29 | 1.08 | 0.92 | 1.01 |
| North Carolina, and Virginia south of Chesapeake Bay | 40 | 1.25 | 0.16 | 0.32 | 3.18 | 1.14 | 1.41 | 1.71 | 0.38 | 1.14 |
| South Carolina and Georgia | 9 | 0.30 | 0.16 | 0.21 | 1.54 | 1.10 | 1.31 | 1.19 | 0.96 | 1.10 |
| Florida | 19 | 0.69 | 0.13 | 0.34 | 1.76 | 1.17 | 1.42 | 1.33 | 0.86 | 1.04 |
Sediments found upon the shallower parts of the continental shelves and upon ridges and banks near shore do not differ appreciably from those found on the beach. In many localities there is a decrease in median diameter on leaving the beach, but such changes are by no means regular. This has been emphasized by Shepard and McDonald (1938) and by Revelle and Shepard (1939) for sediments on the Pacific coast, and by Stetson (1938) for sediments on the Atlantic coast of the United States. In Santa Monica Bay, California, Shepard and McDonald found a marked decrease in median diameter in shallow water within a few hundred feet of shore but no correlation between particle size and depth for the bay as a whole (fig. 262). Stetson's data from the shelf on
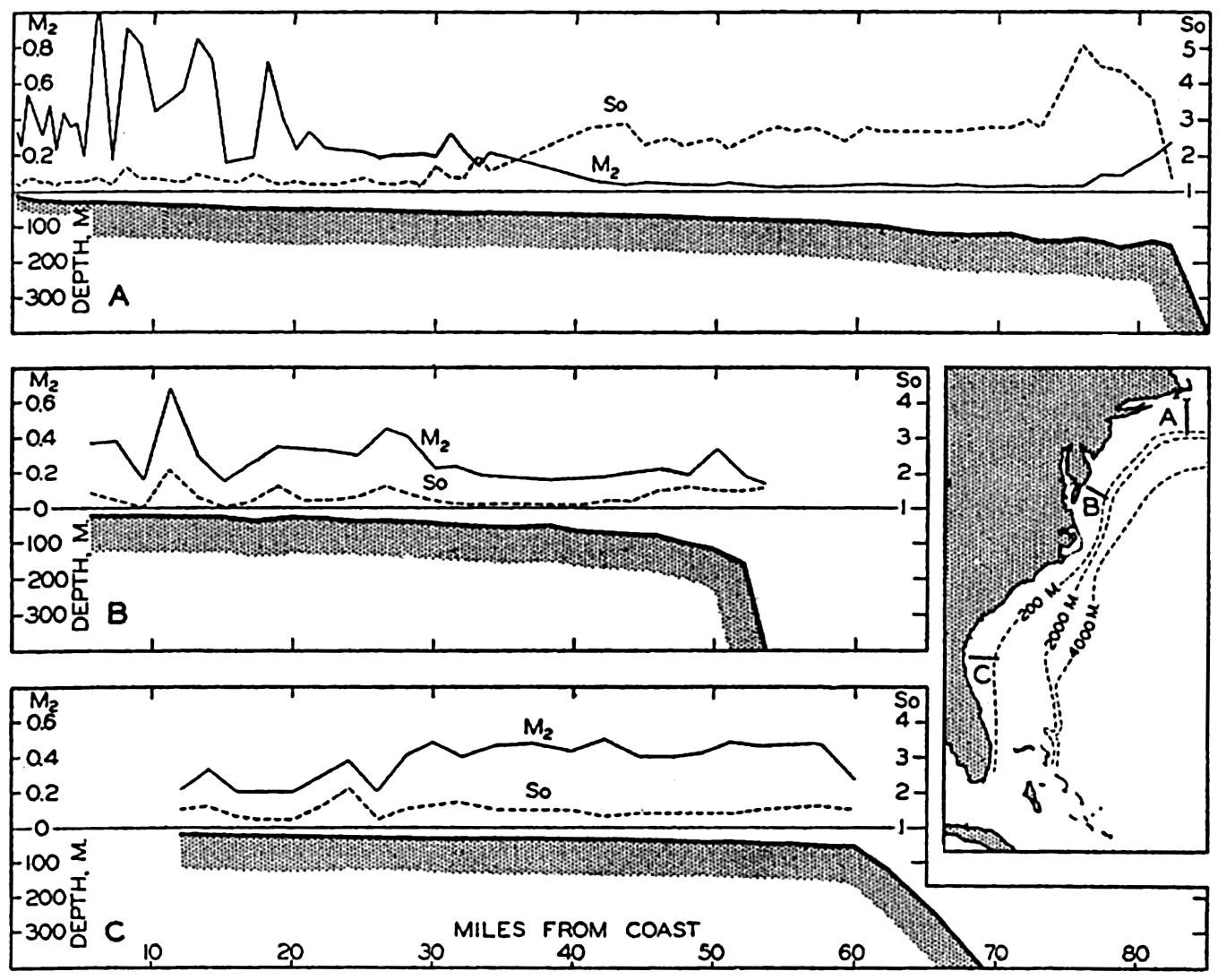
Median diameters in millimeters and sorting coefficients along representative sections off the east coast of the United States in relation to the bottom profile. (Data from Stetson, 1938.)
According to Stetson (1938, 1939) the past geological history and the supply of materials, as well as depth, are important factors in determining the character of the shelf sediments. Off the northern part of the Atlantic seaboard, where glaciation extended out beyond the present coast line, the sediments near shore are relatively fine and well-sorted sands; outside of these are coarser well-sorted sands and gravels; and succeeding these, poorly sorted silts and clays. At the edge of the shelf there is again an increase in the size of the particles. Off Maryland and New Jersey the sediments are well-sorted sands out to the break in slope, and there are no silts and clays. This is ascribed to the lack of a local supply of the finer grades. South of Cape Hatteras the relatively fine, well-sorted inorganic deposits grade into well-sorted calcareous sands
Hough (1940) has emphasized the lack of correlation between median diameter and depth for sediments in Buzzards Bay. However, he found reasonably good correlation between the median diameter and the sorting. Sediments with median diameters greater than 0.15 mm generally had sorting coefficients between 1.25 and 2.0, whereas the coefficients for the finer sediments varied between 2.0 and 3.0
The relatively coarse-grained texture of beach and shelf deposits has an indirect effect upon the composition, since only those materials occurring in relatively hard form and of sufficient size are present as “permanent” constituents of the sediments. Furthermore, as the particles are larger they are not subject to wide dispersal and, hence, are generally found relatively near their site of origin or entrance to the sea. Along irregular indented coasts where there are different types of source materials there may be marked differences in the composition of the beach sediments in adjacent bays. Where it has been possible to trace the dispersal of certain characteristic minerals (Martens, 1939) it has been found that they may travel 200 km or more along an uninterrupted coast. In the absence of relatively large supplies of inorganic material, the skeletal remains of planktonic or benthic plants and animals form a greater or less fraction of the deposit. Organic siliceous remains are never abundant, although they are cosmopolitan in distribution. In general, benthic remains are more abundant than planktonic, although on the outer part of the shelf and on isolated highs, planktonic foraminifera may form an important part of the sediment. The most common remains of benthic organisms are those of calcareous algae, mollusks, foraminifera, corals, bryozoa, annelids, echinoderms, and sponges. Siliceous diatom frustules are abundant in the sediments in some localities. The character of the inorganic fraction is influenced by the nature of the supply. Quartz is the most characteristic material with feldspar and the ferromagnesian minerals common in higher latitudes, particularly off glaciated coasts. Around volcanic islands fragments of andesitic and basaltic lavas with little or no quartz may be most common. The heavy minerals are frequently concentrated on the beaches by sorting action of the waves. The common heavy minerals are ilmenite, magnetite, garnet, zircon, monazite, and olivine (Martens, 1939).
Decomposable organic matter is generally low in the coarser deposits (p. 1016), but in the finer sediments, particularly those in basins, exceptionally high amounts are found. The low content of the sands and silts is ascribed to the fact that the organic matter is more readily attacked in such sediments and also that as the organic matter is light it is readily transported away by currents and deposited in depressions.
Beach and shallow-water sediments range from white to black with the addition of green, red, or blue. The coarser deposits are generally light in color unless in reducing environments. The finer deposits are commonly green, brown, or “blue.” Fine deposits in stagnant environments are black.
Character of the Deposits. In the foregoing discussion, emphasis has been placed on the composition of individual samples although frequent reference has been made to the variability in vertical and horizontal distribution. In fig. 263 were shown examples of the variability in the sediments off the Atlantic coast of the United States. Such differences are mostly in texture and composition, although color as related to either of these or to the environment may be very conspicuous.
Many studies of variability in the properties of beach deposits have been made in order to determine the relative importance of the numerous processes which may influence the character of such deposits. Longshore studies, such as those summarized by Martens (1939) and the detailed investigations of Schalk (1938), show marked differences in the character of the sediments. Thompson (1937) has made careful studies of the laminations which are characteristic of the coarser deposits, and Häntzschel (1939) has discussed similar features in the finer tide-flat deposits. The lateral variability can generally be related to the source localities of the constituent materials, and the relative intensity of wave and current action. The vertical laminations due to differences in texture, composition, and color are to be related to fluctuations in intensity of wave and current action associated with such periodic or random phenomena as the tides, the occurrence of storms or changing currents. Such laminations are transitory as the sediments are subject to reworking by the original causative agents. Häntzschel (1939) has pointed out that in tide flats such laminations are common although there is generally a large population of burrowing and mud-eating animals whose activities tend to destroy them. Temporary erosion and changing slopes may result in complicated internal structures in beaches. Whether or not such features exist on the shelf below tide level has not yet been determined, but as waves and currents are effective to considerable depths it is reasonable to assume that these temporary features, characteristic of the instability of the shallow-water zone, are present.
Students of sedimentary rocks lay considerable emphasis on features formed at the surface between the sediment and the overlying water or air. Such surface features are ripple marks, swash marks, rain and bubble marks, cracks, and so forth. Although common on beaches and in shallow water, it should be remembered that surface features are generally transitory and subject to erasure or changes by the next wave, high tide, or storm. It is only when exposed to the air so that the sediment is dried and thus partly consolidated that there is much likelihood
Special Nearshore Environments. The discussion thus far has been largely restricted to the character of the sediments found on the beach and shelf of the exposed coast. There are, of course, many modifications in the processes and, hence, in the character of the sediments which are associated with special local conditions. Such special localities may be placed in two major groups, (1) those environments which are transitional between marine on the one hand and fresh water, concentrated brine, or terrestrial, on the other hand, and (2) those which are more or less typically marine but where the topography introduces peculiar conditions. Of the latter group, isolated basins are the most important.
Where rivers enter the sea there is a transition from the fresh-water to the marine environment that will be reflected in the character of the fauna and the flora. Within the lower part of a river the tidal effect may be felt and certain parts of the bottom may be alternately exposed to fresh and salt water. This imposes limitations on the nature of the organisms which can develop there. In estuaries and off the mouths of large rivers there may be rapid deposition of the inorganic material carried by the rivers. Russell and Russell (1939) have reviewed the character of the sedimentation processes in the delta of the Mississippi River. The peculiar properties of these and other transitional environments, such as lagoons, have been discussed by Twenhofel (1939). Partially enclosed bays and seas will have beach and shallow-water deposits that may have properties differing to a greater or less degree from those of the open coast depending upon their size, tidal range, strength of waves, frequency of storms, and the character of the materials supplied.
Topographic features of the shelf play an important part in determining the distribution of sediments. The sediments in submarine canyons are of particular interest because of the fact that these channels may be the routes followed by large amounts of sediments which cross the shelves. Cutting as they do far into the shelf, the canyons must entrap large amounts of material in transit along the coast. In the canyon there are no known agencies of sufficient strength to raise the material again to the shelf proper. Near the canyon heads there are rapid and extensive changes in depth, indicating fill and removal. The texture of the sediments in the bottom of the canyons has been studied by Stetson (1939) and by Cohee (1938). Near the canyon heads the materials are commonly slightly finer than those on the adjacent slopes. On the other hand, pebbles and rock fragments have been found on the canyon bottoms. The coarser material may represent the more or less permanent deposit, whereas the fine materials are those being by-passed to the open sea.
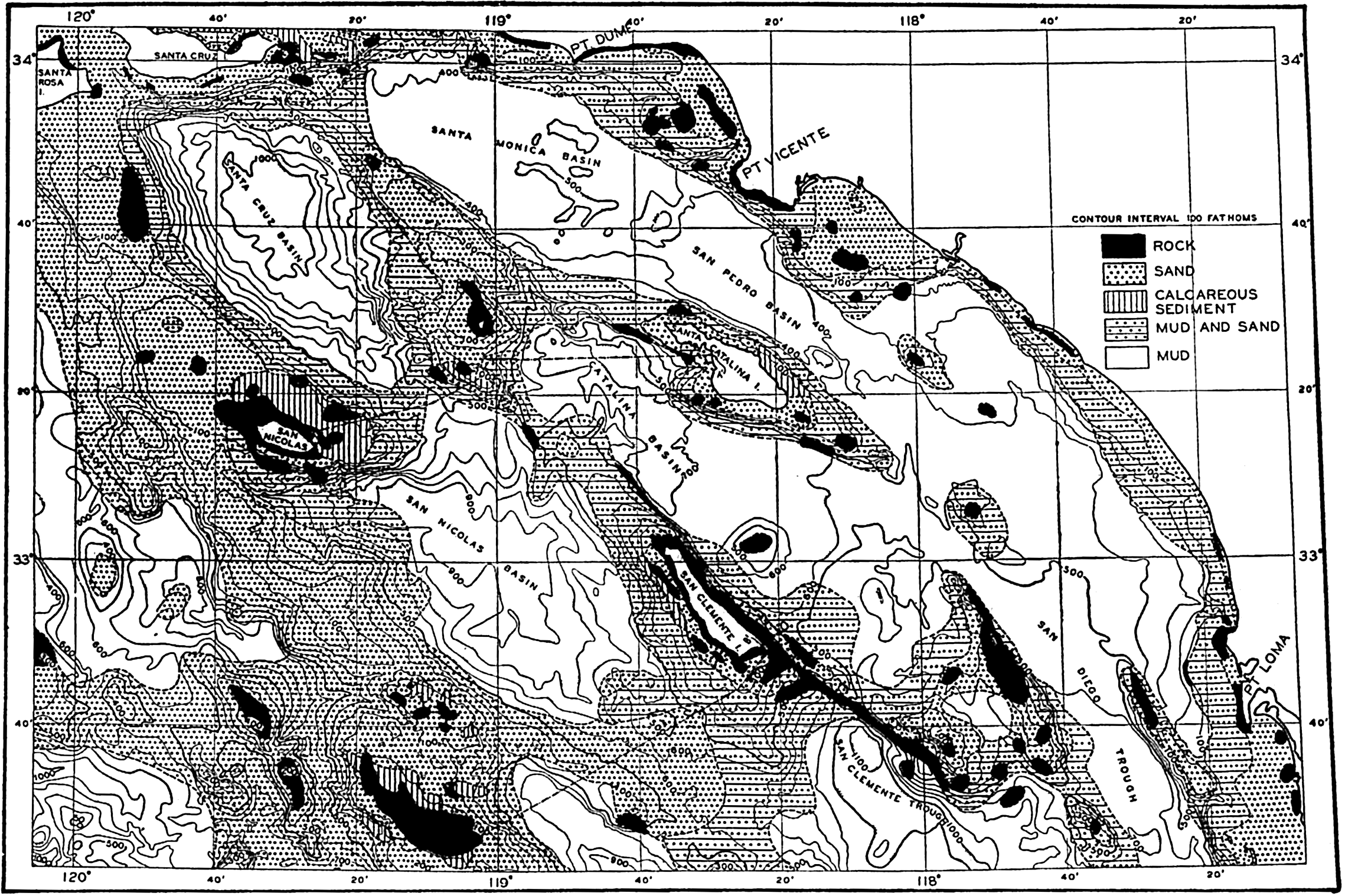
Generalized map of the distribution of rock bottom and sediments off the coast of southern California. Depth contours in fathoms. (From Revelle and Shepard, 1939, in Recent Marine Sediments, P. D. Trask, ed. American Association of Petroleum Geologists, Tulsa.)
Topographically isolated basins are of particular interest because they are one of the environments in which sediments must be accumulating. Basins may be classified hydrographically, depending upon whether the inflow from the open ocean occurs at the surface or at sill depth, or conversely, whether the outflow is at the sill depth or the surface (chapter IV). Biologically and geologically the greatest distinction is between those basins which contain dissolved oxygen in the waters overlying the bottom and those which are stagnant and contain hydrogen sulphide. The latter condition may exclude animals which might otherwise inhabit the water and the bottom and which would contribute to or modify the character of the sediment.
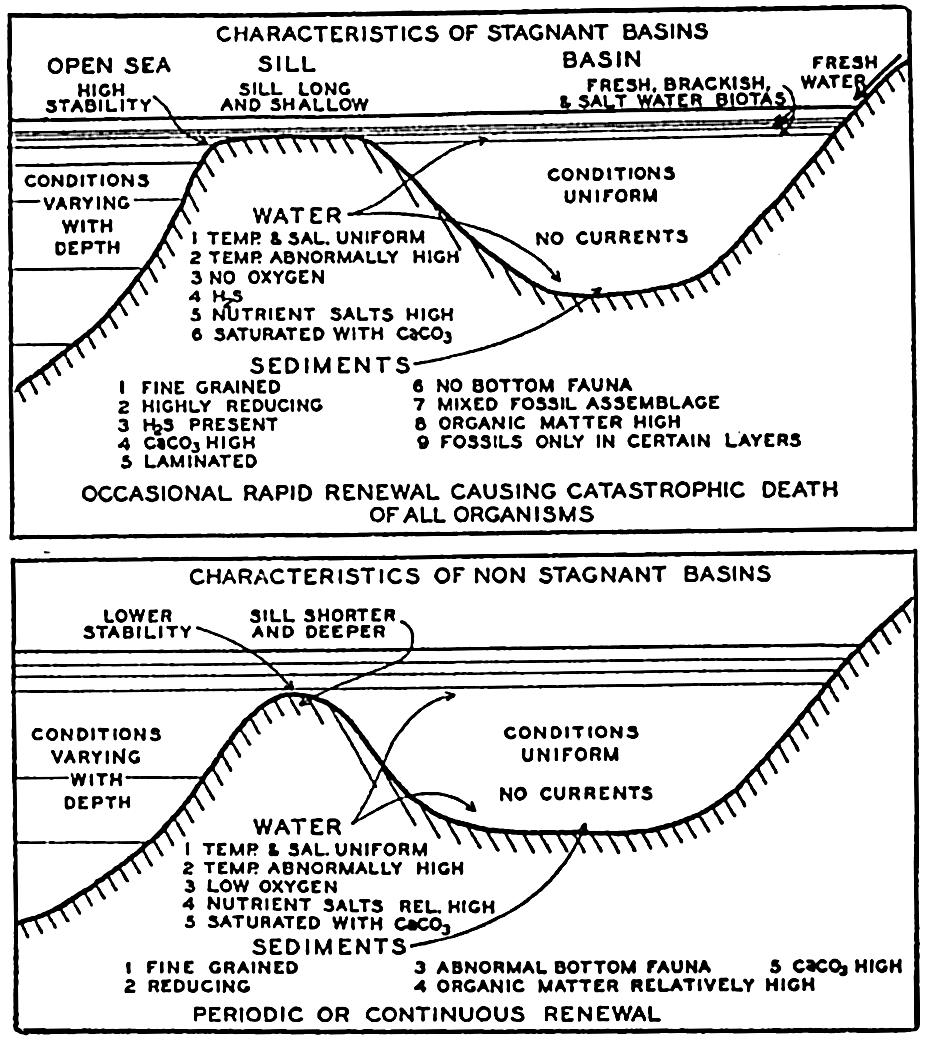
Characteristic features of stagnant and nonstagnant basins. (From Fleming and Revelle, 1939, in Recent Marine Sediments, edited by Parker D. Trask and published by the American Association of Petroleum Geologists, Tulsa, Oklahoma.)
The texture of basin sediments is generally fine but they are poorly sorted. Studies by Revelle and Shepard (1939) in basins off the southern
Fleming and Revelle (1939) summarized Ström's data for stagnant and nonstagnant fjords (see table 118) and showed the characteristic properties of the water and the sediments in such environments (fig. 265).
ELEMENTS CONCENTRATED ON THE SEA BOTTOM AND AUTHIGENIC MINERALS
Certain elements such as calcium, carbon, silicon, iron, and manganese, carried to the sea in solution, are precipitated by organic or inorganic processes and are thereby concentrated on the sea floor. Readily recognizable minerals such as calcite and aragonite are produced in some instances, where in others the concentration is detectable only by chemical analyses which reveal the abnormal content of such elements as iron, manganese, and even the radioactive elements. The interaction of sea water and inorganic debris gives rise to certain characteristic minerals such as palagonite and phillipsite and it is probable that clay minerals are formed from the disintegration of volcanic ejecta (Bramlette and Bradley, 1940). The origin of glauconite which is a common constituent of terrigenous sediments is as yet uncertain, one theory being that it is built up from simple substances, the other that it is a product of the chemical weathering of biotite on the sea floor.
The factors affecting the deposition of calcium carbonate, both as calcite and aragonite, have been described elsewhere. Dolomite has been reported as a rare authigenic constituent of pelagic sediments by Correns (1937). Silicon in the form of dissolved silicates or as colloidal silica is one of the abundant constituents of river water. It is commonly believed that much of the silicon is immediately deposited on entering the sea by precipitation or coagulation when the river water mixes with sea water (Twenhofel, 1939, p. 373). However, there is no evidence for the presence of inorganically precipitated silica in recent marine sediments and the deposition of organic remains must be sufficient to balance the supply of dissolved and colloidal silicon compounds to the sea. The factors governing the deposition of organic siliceous remains have not been investigated thoroughly, but a relatively high content of dissolved silicates
Manganese is one of the chemical elements which is present in red clay in greater concentration than it is in igneous rocks (table 111, p. 992). In marine sediments it is chiefly present as oxides, largely manganese dioxide (MnO2), and may exist as finely divided grains, coatings over shells and inorganic material, as a cementing matrix, and in nodules and concretions. Manganese nodules, having diameters of 10 cm or more, are conspicuous constituents in dredge and trawl samples and have aroused great interest since the Challenger investigations showed their rather wide distribution. Although the nodules are characteristics of the deep-sea sediments, manganese in its other forms is commonly found also in terrigenous sediments. The manganese concretions and nodules which are found most abundantly in the South Pacific and Indian Oceans contain variable proportions of manganese dioxide, ferric oxide (limonite), and clay. The average contents of MnO2 and Fe2O3 are about 29.0 per cent and 21.5 per cent, respectively. Many individual analyses are given by Murray and Renard (1891) and by Andrée (1920). The nodules generally show laminations of different shades and textures and have a roughly concentric structure with a nucleus of pumice, volcanic glass, rock, or organic material such as a shark's tooth or an earbone of a whale.
| Characteristic | Stagnant fjords | Nonstagnant fjords |
|---|---|---|
| Amplitude of spring tides (cm) | 44 | 41 |
| Length (km) | 7 | 7 |
| Width (km) | 1.5 | 1.2 |
| Maximum depth (m) | 102 | 119 |
| Sill depth (m) | 4.2 | 10.8 |
| Surface temperature, °C | 16.3 | 16.6 |
| Bottom temperature, °C | 6.3 | 5.6 |
| Depth of isothermal layer (m) | 26 | 44 |
| Surface salinity (‰) | 19.54 | 23.10 |
| Bottom salinity (‰) | 30.07 | 33.52 |
| Density difference (surface to bottom) | .0115 | .0100 |
| Bottom H2S (ml/L) | 9.14 | |
| Bottom O2 deficit from saturation value (ml/L) | 11.74 | 5.03 |
| pH | 7.05 | 7.34 |
| P2O5 (mg/m3) | 327 | 67 |
| Organic carbon in muds, per cent | 13.0 | 9.9 |
| Thickness of black, putrid layer (cm) | >40 | ca. 20 |
Although the large nodules have aroused great interest, it is probable that they do not represent the bulk of the manganese present in most sediments. Correns (1937) in his studies of the Meteor material from the Equatorial Atlantic found that the content of manganese was conspicuously higher than in igneous rocks but he was unable to detect any manganese grains. Ten representative red clays from the Atlantic (Correns, 1939) contained an average of 1.2 per cent MnO2 on a CaCO3-free basis. The noncalcareous portion of 19 typical globigerina oozes contained 0.5 per cent MnO2. Additional data are given in table 119 (from Correns, 1939). The dark color of the red clays of the Indian and Pacific Oceans has been attributed to their manganese content, but comparison of the Carnegie data from the Pacific (Revelle, 1936) shows that the red clays of the North Pacific are lower in manganese than those of the Atlantic.
Table 111 (p. 992) shows the average of 10 North Pacific red clays to be 0.83 per cent MnO2, that is, approximately one half that of the Atlantic samples. In contrast to the samples from the Atlantic the MnO2 content is much higher in the calcareous deposits of the Pacific Ocean from which 20 samples containing more than 30 per cent CaCO3 contained on an average 5.5 per cent MnO2 when calculated on a CaCO3-free basis (Revelle, 1936). The great difference between the manganese content in the calcareous oozes of the Atlantic and the Pacific and the fact that there is no correlation with the percentage of calcium carbonate indicate that the deposition is not closely related with the accumulation of calcareous material.
There are two possible sources of the mangenese in deep-sea deposits. Murray and his associates concluded that it was a product of the submarine weathering of volcanic material. However, volcanic rocks do not contain very large amounts of manganese and in order to build up the concentrations found in red clays and in the noncalcareous fraction of globigerina oozes, it would be necessary to have large amounts of silica, aluminum, and iron leached out and redeposited elsewhere, since there is no accumulation of these substances in solution. According to Washington (1920), the average managanese content of the oceanic volcanics, expressed as MnO2, is between 0.05 and 0.18 per cent, the lower value being for the islands of the Atlantic and the higher for those of the Pacific Ocean. Consequently, a five- to tenfold concentration of manganese would be necessary if the clays were formed from this material. The manganese carried to the sea in solution forms an extremely small fraction of the dissolved solids and is rather rarely determined. According to Twenhofel (1926, p. 406) the concentration generally varies between 0.5 and 5.0 mg/L, which is greater than the amount present in sea water, namely, 0.001 to 0.01 mg/L. Murata (1939) has studied the total and exchangeable manganese content of river muds, namely, particulate material which is carried to the sea. The average total manganese in five river muds was 0.18 per cent, as MnO2, and the exchangeable manganese in these river muds was found to range from 0.03 per cent to 0.07 per cent as MnO2. From this it may be concluded that managanese is carried to the sea both in solution and as a constituent of the sedimentary debris.
| Maximum | Minimum | Percentage of calcium carbonate | All samples | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–10 | 10–20 | 20–30 | 30–40 | 40–50 | 50–60 | 60–70 | 70–80 | 80–90 | |||||
a, percentage of total weight of sediments; b, percentage on carbonate-free basis. | |||||||||||||
The rows for the samples represent the number of samples; the other rows represent average percentage of the respective substances in the sediments. | |||||||||||||
| a | 4.00 | Trace | Samples | 14 | 2 | 9 | 6 | 12 | 10 | 18 | 11 | 6 | 88 |
| MnO2 | Average | 0.62 | 0.08 | 0.56 | 0.13 | 0.23 | 0.36 | 0.09 | 0.08 | 0.12 | 0.28 | ||
| b | 5.08 | Trace | Samples | 13 | 2 | 9 | 6 | 12 | 9 | 17 | 9 | 6 | 83 |
| Average | 0.64 | 0.12 | 0.72 | 0.20 | 0.42 | 0.81 | 0.28 | 0.47 | 0.77 | 0.51 | |||
| a | 10.69 | 0.21 | Samples | 14 | 2 | 9 | 6 | 12 | 10 | 18 | 10 | 6 | 87 |
| Fe2O3a | Average | 5.525 | 6.91 | 3.623 | 3.61 | 3.63 | 2.96 | 2.56 | 2.74 | 1.58 | 3.47 | ||
| b | 19.16 | 0.303 | Samples | 13 | 2 | 9 | 6 | 12 | 10 | 18 | 10 | 6 | 86 |
| Average | 5.71 | 8.49 | 4.71 | 5.47 | 6.61 | 6.67 | 7.32 | 10.89 | 9.82 | 7.12 | |||
| a | 1.06 | Trace | Samples | 14 | 2 | 9 | 6 | 11 | 10 | 18 | 11 | 6 | 87 |
| P2O5 | Average | 0.196 | 0.396 | 0.161 | 0.131 | 0.27 | 0.154 | 0.112 | 0.11 | 0.092 | 0.162 | ||
| b | 1.71 | Trace | Samples | 13 | 2 | 9 | 6 | 11 | 9 | 17 | 11 | 6 | 84 |
| Average | 0.257 | 0.489 | 0.21 | 0.1913 | 0.510 | 0.378 | 0.343 | 0.398 | 0.558 | 0.356 | |||
Manganese is most soluble in acid solutions when in reduced state. Hence, environments where such conditions prevail will tend to leach the manganese out of the solid material. Conversely, alkaline and oxidizing environments tend to precipitate the manganese in the very insoluble form of manganese dioxide. Whether or not bacteria or other biological agencies are directly involved in the solution or precipitation of manganese is not definitely known (ZoBell, 1939).
On reaching the sea the dissolved and exchangeable manganese is probably precipitated as finely divided MnO2 suspended in the water. If this settles in terrigenous environments where reducing conditions prevail, it again may pass into solution. The most favorable conditions for accumulation are those where the oxidation-reduction potential is relatively high as on topographic highs and in pelagic deposits where the conditions are always oxidizing, and in the presence of even slight amounts of solid CaCO3 where the pH must be relatively high. Hence, once the manganese is deposited in such an environment it is not likely to pass into solution again. This explanation does not account for the formation of the large manganese nodules which, according to Murray and Renard, may occur in clay which itself is relatively low in manganese. However, it is known that manganese accumulations tend to form on similar material, somewhat analogous to crystal growth, and possibly this may be one of the important factors. The difference in the manganese content of the sediments of the equatorial Atlantic and those of the Pacific Ocean is a problem which is yet unsolved.
Iron (table 111, p. 992) is present in red clays in concentrations somewhat greater than in the igneous rocks and terrigenous deposits, although the increase is not as marked as for manganese. As pointed out above, the manganese nodules contain a large proportion of iron oxides. Authigenic iron compounds may be found as the oxides, principally limonite and as sulphides. Table 119 contains data on the iron content of the Meteor samples, which, computed on a CaCO3-free basis, is rather erratic, and shows no correlation with the content of organic remains. The average iron content, calculated as Fe2O3, is 7.12 per cent, which, as in the case of manganese, is less than the average for the red clays. This Correns attributes to the higher iron content of the deposits of the Pacific and Indian Oceans. Examination of the Carnegie data shows that the samples with less than 30 per cent CaCO3 calculated on a CaCO3-free basis contain 7.0 per cent iron calculated as Fe2O3, whereas the calcareous deposits computed in the same way contain, on an average, 15.6 per cent
Two sources may supply iron to the marine sediments. These are: (1) the weathering of the oceanic volcanic material, which is relatively high in iron as indicated by the analyses of Washington (1920), which give about 10.8 per cent iron, calculated as Fe2O3; and (2) the considerable amounts which are carried to the sea in solution and as a constituent of the mineral debris. As the latter material may be relatively low in iron it may have a diluting effect and the dissolved iron must be the important source. Acid and reducing conditions tend to dissolve iron compounds, while alkaline and oxidizing conditions tend to precipitate the higher oxides. A mechanism somewhat similar to that described for manganese may therefore apply to the iron. The most favorable environment for the accumulation of iron oxides will be in oxidizing sediments containing calcareous material. Iron is precipitated as sulphides in stagnant environments where hydrogen sulphide is produced. Bacteria may be directly involved in the solution and precipitation of iron oxides and sulphides (Twenhofel, 1939).
Phosphorus, as P2O5, (table 111, p. 992) is found in approximately the same amounts in the red clays as in igneous rocks. However, Correns’ data (table 119) indicate that on a CaCO3-free basis there is an increase in P2O5 with the carbonate content indicating that the phosphate may form about 0.1 per cent of the calcareous skeletons. The supply of dissolved phosphate and its organic cycle in the sea have been discussed elsewhere. Apparently the regeneration of phosphorus utilized by plants is relatively complete, but it is being added to the sea as dissolved phosphate as well as a constituent of the minerals, chiefly apatite. The supply of dissolved phosphorus may be balanced by the removal in skeletal structures, some of which are extremely high in phosphorus. Furthermore, a certain amount may be deposited as a constituent of the detrital organic matter. Authigenic phosphorus compounds are not found in the pelagic deposits, but in certain nearshore localities phosphorite, Ca3(PO4)2, forms a cementing material which accumulates in nodules and crusts. These phosphate nodules are first discovered by the Challenger off the Cape of Good Hope and have subsequently been found in many coastal regions on the edge of the continental shelf and on topographic highs. Dredging off the southern California coast has shown phosphorite to be the most abundant type of rock collected.
The material collected off the coast of southern California (Deitz, et al, 1942) contained phosphorite nodules ranging in size from small oolites to
The phosphorite nodules have a characteristic smoothly rounded surface with the upper surface having a glazed unweathered appearance. Freshly broken surfaces are light brown to black and the outer surface is generally somewhat darker, owing to a thin coating of manganese oxides. Microscopic and chemical analyses show the principal mineral to be collophane. This may form virtually pure masses or act as a cement for phosphatic oolites and inorganic and organic material some of which may be more or less completely phosphatized. Most of the nodules show banding, indicating that the accumulation has been discontinuous. The calcium phosphate on the average forms about 67 per cent of the nodules, with calcium carbonate the second most abundant constituent. Sediments in the vicinity of phosphorite deposits contain approximately the same amounts as given above for pelagic deposits.
The phosphate nodules appear to be forming at the present time and several hypotheses have been advanced to explain their mode of formation (Twenhofel, 1939, Dietz et al, 1942).
Barium sulphate concretions have been found in recent marine sediments only in three widely separated localities, namely, off the west coast of Ceylon (1235 m), near the Kai Islands in the Dutch East Indies (304 m), and near Catalina Island off the coast of California (800 to 650 m). The concretions, ranging in weight from a few grams to about 1 kg, are generally of irregular shape, sometimes tubular, with concentric banding. They contain from about 60 to 82 per cent barium sulphate, which forms a cementing matrix, and contain mineral grains as well as organic remains. The bulk density of the barite concretions is high, averaging 2.70 and sometimes exceeding 3.0. Emery and Revelle (1942) examined the California material and consider that the only explanation which will account for their characteristic properties and their restricted occurrence is that the concretions are formed in the mud by the interaction of hot spring water high in barium with the sulphate present in the sea water.
The radioactive elements, particularly radium, in marine sediments have received considerable attention because of the unusually high radium content of pelagic sediments and because of the possibility of using the changes with depth in cores as a means of establishing the rates of deposition. Existing data have been assembled by the principal workers in the field, namely, Evans and Kip (1938), Evans, Kip, and Moberg (1938), Utterback and Sanderman (1938), Föyn, Karlik, Pettersson, and Rona (1939), and Urry and Piggot (1941).
The radium content of marine sediments varies between 0.1 X 10−12 g/g and values more than one hundred times as large. Low values are found in coarse-grained terrigenous deposits, whereas the high values occur in noncalcareous pelagic sediments. Bottom samples collected in the Pacific Ocean by the Carnegie were examined by Piggot and the average radium contents of the various types of sediments are given in table 120. (Revelle, manuscript.) Nearshore sediments, such as those studied by Utterback and Sanderman (1938), average about 0.3 X 10−12 g/g of radium.
The radium content of granites is about 1.6 X 10−12 g/g and that of basalts even less. The higher radium content of deep-sea marine sediments must therefore be due to a concentration taking place on the sea floor. Radium has a relatively short half-life period of about 1600 years. If radium alone were deposited on the sea floor where the rate of sedimentation is less than 1 cm in a 1000 years the amount present in the lower parts of cores, a meter or more in length, would be extremely small. Although there is generally a decrease in radium with depth, the distribution (cf. Urry and Piggot, 1941) shows that other radioactive elements which disintegrate to form radium must be deposited. Of these ionium and possibly uranium are of importance.
| Type | Number of samples | Radium g/g |
|---|---|---|
* Relatively high in siliceous organic remains. | ||
| Radiolarian ooze | 3 | 14.1 X 10−12 |
| Red clay | 7 | 8.7 |
| Globigerina ooze (siliceous[*]) | 4 | 7.2 |
| Diatom ooze | 3 | 5.0 |
| Globigerina ooze | 8 | 3.7 |
| Terrigenous mud | 2 | 2.5 |
Various mechanisms by which radium and the other radioactive elements may be concentrated in marine sediments have been suggested. Coprecipitation of radium with calcium carbonate and of uranium with iron and manganese compounds has been offered as possible mechanisms, but such hypotheses are not substantiated by the available data. Revelle (1936) pointed out the correlation between the radium and the decomposable organic matter in the Carnegie samples and suggested that organic activity might be the effective precipitating agency. Subsequently (Revelle, manuscript) he has developed this hypothesis and shown a correlation between the radium content and the organic siliceous remains
Glauconite is a green, blue, or brown hydrous silicate of potassium, magnesium, aluminum, and ferrous and ferric iron. The formula for glauconite can be written R2′O, 4(R″O,R2‴O3),10SiO2,4H2O, where R′ represents the univalent bases and R″ and R‴ the di- and trivalent bases. It is found in recent marine sediments and in many marine sedimentary rocks and it is considered to be a substance formed only in the marine environment. The distribution and properties of glauconite in both recent and fossil deposits and the various theories which have been advanced to explain its formation have been reviewed by Hadding (1932), Takahashi (1939), and Galliher (1939). Analyses given by Hadding and Takahashi show that although there is a considerable range in chemical composition there is no essential difference between the recent and the fossil material. However, as Takahashi has pointed out, the chemical composition can be varied considerably by treatment with neutral salt solutions as well as with acid and alkaline solutions. As the X-ray spectra are not materially different, it is probable that the exchange of cations is somewhat analogous to base-exchange in clay minerals.
Glauconite occurs in sediments as irregular grains between 0.1 and 1.0 mm in diameter. The surface is often polished and sometimes cracked and the grains have no definite internal structure. When present in sufficient quantity they give a greenish color to the sediment and the terms green sand and green mud were originally restricted to sediments containing glauconite. The glauconite grains often represent casts of foraminiferal shells and, in some cases, the shells are found completely filled by glauconite. Hadding has recorded glauconite present as interstitial material in porous organic and mineral substances and also as an incrustation.
Glauconite occurs in recent terrigenous sediments off continental coasts at depths ranging from a few meters down to about 2500 m. According to Galliher, it is more abundant adjacent to land areas where plutonic and metamorphic rocks are exposed. Although glauconite is found in many parts of the world it is abundant only in rather restricted localities where the environment is appropriate for its formation or accumulation, where deposition is not rapid, namely, upon the continental shelf or isolated topographic highs near the coast.
The mode of formation of glauconite is not yet definitely established. The older theories postulate the formation of an aluminosilicate gel which
RATES OF SEDIMENTATION
Methods of Determining Rates of Sedimentation. Knowledge of the rates of accumulation of sediments in the ocean is essential to an understanding of many problems both of past geological history and present sedimentation processes, but unfortunately very little is yet known about these rates. In the following discussion various indirect methods of estimation will be listed, all of which lead to values of approximately the same magnitude. The development of new means of determining the time scale, such as those based on radioactivity and magnetic measurements, may ultimately produce more reliable values. The rates of deposition are extremely small, in the open ocean of the order of less than 1 cm per 1000 years, yet, as pointed out by Kuenen (1937) and others, it is difficult to account for the supply of the tremendous mass of material which must have accumulated in the deep sea since the beginning of geological time. Questions concerning the total quantity of marine sediments, not entered upon here, have been discussed by Clarke (1924), Kuenen (1937, 1941), and Goldschmidt (1933).
Because of the extremely slow rate of deposition of deep sea sediments no adequate direct method of measurement has yet been devised. We are therefore forced to rely upon indirect methods which fall in two general groups. The stratigraphic method, widely used in the study of sedimentary, rocks, may be applied when it is possible to determine the thickness of the deposit which has accumulated in a given time interval. The supply method for estimating the rate of deposition is applicable when
The difficulty of obtaining adequate cores represents the greatest obstacle to the wide application of the stratigraphic method to recent marine sediments. Not only is it a question of obtaining cores of sufficient length but also of avoiding as far as possible the effects of what is known as “compaction” due to unsatisfactory sampling methods. Also it is often impossible to establish the time scale. To make the figures comparable to those obtained by other methods the length of core must be computed for a constant pore space (water content) or the values may be given in terms of solid material. This is important because superficial layers of sediments consist of about two thirds by volume of water. In the following discussion values are given for a “solid” layer of density 2.5, that is on a pore-free basis.
The supply method is based upon the rate of supply of sedimentary material to the sea. Such material may arise from submarine or subaerial volcanism, from erosion and transport, either in solution or in suspension, of terrestrial material, and contributions from outer space. The latter source is negligible but the magnitude of submarine volcanism is unknown. Furthermore the amount of air-borne materials, both of volcanic and terrestrial origin, has commonly been neglected, although, as shown elsewhere (p. 949 to p. 955), these may form appreciable fractions of pelagic deposits. Indirect estimates of the rate of sedimentation can best be based on the supply of river-borne dissolved material, since the amount of solid undissolved material carried to the sea by rivers is not known (Twenhofel, 1939). Rates based on the supply of dissolved material must be fairly accurate because the bulk of the elements are probably
Estimates Based on Stratigraphic Methods. The stratigraphic method has been widely applied to the study of fossil sediments, but the results obtained are very variable. Average values for sediments deposited since the Cambrian (500,000,000 years) have been derived by a study of the thickness of the column of sediments using a time scale based on radioactivity measurements. These values are (Schuchert, 1931):
| Sandstone | 68 cm/1000 years |
| Shale | 34 cm/1000 years |
| Limestone | 14 cm/1000 years |
These are for shallow water deposits where the rates of accumulation must be much greater than in the open sea.
The stratigraphic method was first applied to recent deep-sea sediments in 1913 by Braun, whose data have been re-examined by Schott (1939a). For many years it has been recognized that sediments from various parts of the deep sea are stratified. The development of more adequate coring instruments, capable of obtaining cores up to 3 m in length, has opened a new field in the study of these deposits. The interpretation of textural and other differences in these sedimentary columns in deep-sea core samples was expanded by Schott (in Correns, 1937), who examined the systematic series of samples collected by the Meteor in the Equatorial Atlantic. He found that there were characteristic differences in the pelagic foraminiferal fauna at different depths in the cores. Specifically, various warm-water forms were not found in certain zones in the column. The absence of these species was attributed to the cooler climatic conditions and possibly the modified circulation during the glacial periods. The thickness of the superficial layer, containing warm-water forms, was considered to represent the deposition during the time interval since the last glacial period, which Schott assumed to be 20,000 years. On this basis he calculated the rate of accumulation of globigerina ooze, blue mud, and red clay in the Equatorial Atlantic. Schott's values computed from the observed length in the core sample and not corrected for water content or for any distortion or compaction in sampling, are given in table 121. The average values for “blue” mud, globigerina ooze, and red clay, namely 1.78, 1.2, and 0.86 cm per 1000 years become 0.59, 0.4, and 0.29 cm per 1000 years if reduced to 1/3 to place them on a pore-free basis. The figures for globigerina ooze and diatom ooze in the southern Indian Ocean based on Braun's data are somewhat smaller. Uncorrected, the averages are 0.59 and 0.54, respectively, and if reduced to 1/3, 0.20 and 0.18 cm per 1000 years. The rate of deposition of globigerina ooze in the southern Indian Ocean is apparently about one half that in the Equatorial Atlantic.
A series of cores, obtained by means of the Piggot coring tube across the North Atlantic between the Newfoundland Banks and Ireland, have been examined (Bramlette and Bradley, 1940). In certain of these cores, ranging up to 3 m long, systematic variations in the character of the material with depth in the cores were found; that is, strata were found that contained coarse-grained material that could only have been transported to the site of deposition by floating ice. These strata were laid down during the glacial periods. Bramlette and Bradley (1940) state that in cores from the western North Atlantic the postglacial sediments are approximately 34 cm thick, which is within the range found for the Meteor samples.
| “Blue” mud | Globigerina ooze | Red clay | |
|---|---|---|---|
| Average thickness (cm) | 35.5 | 24.06 | 17.14 |
| Greatest observed thickness (cm) | 66.0 | 42.5 | 26.5 |
| Smallest observed thickness (cm) | 18.0 | 10.5 | <10.0 |
| Average rate of sedimentation (cm) per 1000 years | 1.78 | 1.2 | <0.86 |
| Greatest observed rate of sedimentation (cm) per 1000 years | 3.3 | 2.13 | 1.33 |
| Smallest observed rate of sedimentation (cm) per 1000 years | 0.9 | 0.53 | <0.5 |
| Samples used (number) | 6 | 48 | 7 |
From the foregoing it might be concluded that present-day coring techniques would show all pelagic samples to be stratified, but this is not necessarily the case. The absence of stratification within the upper few meters, the present limit of core length, may be due to a number of factors such as: more rapid local accumulation than indicated by the values given above; extensive mixing and churning of the deposit by current action, or the activities of burrowing and mud-eating benthic animals, or the absence of agencies of sufficient magnitude to modify the character of the material being deposited. The latter condition may prevail for such deposits as red clay.
The stratigraphic method has not yet been widely applied to the study of recent shallow water sediments. Moore (1931) estimated the rate of deposition in the deeper portions of the Clyde Sea from laminations which he interpreted as due to the deposition of large amounts of organic matter each spring. The laminations were between 6 and 4 mm thick, the thicker layers occurring in the upper portion of the core. The water
Estimates Based on Supply Methods. Clarke (1924) used his figures for the annual contribution of dissolved materials by rivers (p. 214) to estimate the rate of “chemical” sedimentation. He assumed that only sodium and chlorine escaped deposition by either inorganic or biological processes, and in this way found that the annual rate of chemical deposition was between 22 X 108 and 24 X 108 metric tons. If evenly spread over the entire sea floor this would form a layer of 0.25 cm/1000 years. To this must be added the particulate material of terrigenous and volcanic origin. Furthermore, it has been pointed out that nearshore deposits accumulate more rapidly than those in the open sea; therefore the value given above is too small for terrigenous deposits and too large for the deep sea sediments.
Kuenen (1937), from a consideration of the extent of erosion since the Cambrian (500,000,000 years) and the supply from terrestrial volcanoes, has estimated that the combined chemical and detrital deposition in the deep sea has been at a rate of about 0.33 cm/1000 years, a value approximately the same as those given above. Since then, Kuenen (1941) has revised his figures and reduced them to 0.1 cm/1000 years for red clay and to 0.2 cm/1000 years for globigerina ooze.
Two estimates have been made on the basis of organic deposition. Murray (Murray and Hjort, 1912) estimated the rate of deposition of globigerina ooze as 2.5 cm/10 years. This highly erroneous value was based on the study of the Atlantic submarine telegraph cables which were found to be “covered” in a period of 10 years. Actually they undoutedly settled into the ooze when laid. Computed in the units given above, this would be about 100 cm of solid material per 1000 years. Lohman estimated the rate at which coccoliths might accumulate on the sea floor from his studies of the production of the planktonic calcareous
Revelle and Shepard (1939) have used the supply method for estimating the rate of deposition in the basins of the southern California region. Their value, based on the rate of erosion of the local watersheds and the relative areas of erosion and deposition, is approximately 25 cm/1000 years.
The further development of the methods outlined above and the introduction of new techniques will undoubtedly tend to modify the values for the rates of deposition, but it does not seem probable that they will affect the order of magnitude of those for the deep sea. Large local variations in the rate of deposition of terrigenous depositions must be expected but the scattered evidence indicates that they accumulate at a rate of the order of 10 cm of solid material per 1000 years.
SUMMARY OF FACTORS DETERMINING CHARACTER OF MARINE SEDIMENTS
It has been repeatedly emphasized that the factors controlling the character of the sediment in a given locality are numberous and complicated, and it is both instructive and valuable to see to what extent it is possible to predict the characteristics of the sediment which may occur under a given set of conditions. The number of variables necessary to formulate such a prediction indicates the large number of factors which must be taken into account. The prediction of the mass properties of a sediment for a given set of conditions not only illustrates that we have some understanding of the processes of marine sedimentation but that the procedure may be reversed and certain of the important environmental factors deduced from the properties of a given sediment sample. This is of obvious value to the student of sedimentary deposits both recent and fossil, and is also instructive to the oceanographer concerned with aspects of the sea other than the sediments.
It is difficult to designate those properties of a sediment which are most “important.” The relative importance may vary with the individual interest of the worker, and what in one instance may be a minor constituent of a sediment may in another locality be the most abundant or conspicuous type of material. Glauconite in an example of such a substance. As a basis for the following discussion, those mass properties used to classify and name marine sediments will be considered, namely color, physical composition, and texture.
The environmental factors most important in determining the characteristics of marine sediments may be grouped under three main headings: (1) the general topography and depth of the site of deposition, (2) the relation of the site of deposition to sources of inorganic material, and (3) the physical and chemical conditions in the water column overlying
Topography has its more profound influence upon the textural characteristics of the sediments, and the topography surrounding a given site is usually more important than the absolute depth. Topographic highs are subject to the sweeping action of waves and currents and, hence, are either covered by relatively coarse material or lacking in unconsolidated material. Fine-grained material is always absent in shallow water except on the continental shelf, where it is being by-passed to deeper water. Conversely, depressions and basins generally contain fine-grained material but in addition may receive some coarse material swept off the shelf and highs, or supplied by pelagic or benthic organisms. The slopes where active deposition is taking place are generally covered with poorly sorted sediments. The rate of sedimentation is small on isolated topographic highs and in the major ocean basins and is relatively great on and immediately below the slopes and in nearshore basins. In those areas where the bottom is sinking, accumulation may be rapid on the shelves, otherwise it is negligible. The effect of topography and depth on texture is related to the fact that the strongest water movements, hence the ability to move particles, occur near the surface, particularly at those depths where wave action is effective. Isolated highs at all depths are affected by the sweeping action which is a combination of current motion and the force of gravity which tends to pull the material down slopes.
Indirectly topography may affect the composition where two or more types of materials have their greatest abundance in different size grades. In this case the material having the coarser texture will be concentrated on areas subjected to the stronger transporting agency, with the resulting increased relative concentration of the substance of finer texture elsewhere. This factor may account for the abundance of such materials as glauconite, phosphorite, and these materials mixed with organic remains on certain areas of the shelves, particularly on topographic highs. It has also been advanced as a possible reason for the lower calcium carbonate content of the sediments in the bottom of the major ocean basins where the finest inorganic debris accumulates.
The physical composition of a sediment sample reflects the relative rates of supply of the various types of material. These constituents fall into two major groups, namely, the organic skeletal structures and the inorganic material that may be of terrigenous or volcanic origin. The amount of inorganic material being deposited at a given locality depends upon the relation of that site to the sources of supply. Off the mouths of large rivers and near regions of active volcanism there are relatively large amounts of coarse-grained clastic material. On the other hand, in the open ocean, far removed from the source of such material, the inorganic
After the inorganic material has reached the sea the effect of transporting agencies must be considered. Because of the decreasing intensity of wave action with depth, there is a tendency for the coarser materials to accumulate near the source, whereas the finer materials are transported for great distances. The character of the inorganic material and the texture of the material in a sediment at a given locality can therefore be predicted with some accuracy if we know the depth and topography of the site of deposition and the character of the source. The relative abundance of such material in a sediment of course depends upon the relative rate of deposition of organic material.
The supply of inorganic material is limited to the coast lines and to regions of submarine volcanism and there are of course regions of the sea which are thousands of miles from any such source. This is not so true
The physical-chemical conditions surrounding topographic highs are generally rather similar to those in the surrounding water at approximately the same depth. This statement does not apply, however, to isolated depressions where basin conditions may prevail. In such regions (p. 1026) the physical-chemical conditions may be very different from those at a comparable depth in the open sea in adjacent regions. The most striking contrasts are found in such stagnant basins as the Black Sea and the Norwegian fjords. Sediments accumulating in basins have certain characteristic features which may be used to identify them.
A prediction of the character of a sediment may therefore be made if the following variables are established: topography surrounding the site of deposition, the depth, the relationship to the sources of inorganic material, and the physical-chemical conditions in the overlying water. Conversely, if the character of a sediment is known it is possible to recognize the more important factors of the environment of deposition.
Bibliography
Anderson, D. Q.1939. “Distribution of organic matter in marine sediments and its availability to further decomposition” . Jour. Marine Research, v. 2, p. 225–235, 1939.
Andrée, K.1920. “Geologie des Meeresbodens” . Bd. II. Bodenbeschaffenheit, Nutzbare Materialien am Meeresboden. Leipzig, Gebrüder Borntraeger, 689 pp., 1920.
Bramlette, M. N., and W. H. Bradley. 1940. “Geology and biology of North Atlantic deep-sea cores” . Part 1. Lithology and geologic interpretations. U. S. Geol. Survey, Prof. Paper 196-A, 34 pp., 1940.
Cayeux, Lucien. 1931. “Introduction à l'étude pétrographique des roches sédimentaires” . Paris, Ministére des Travaux Publics, Texte, 524 pp., Atlas, 56 pls., 1931.
Clarke, Frank W.1924. “The data of geochemistry” . U. S. Geol. Survey, Bull. 770, 841 pp., 1924.
Cohee, George V.1938. “Sediments of the submarine canyons off the California coast” . Jour. Sedimentary Petrology, v. 8, p. 19–33, 1938.
Collet, Léon W.1908. “Les dépôts marins” . Paris, Octave Doin, Éditeur, 325 pp., 1908.
Cornish, Vaughan. 1934. Ocean waves and kindred geophysical phenomena. London, Cambridge Univ. Press, 164 pp., 1934.
Correns, Carl W.1939. Pelagic sediments of the North Atlantic Ocean. p. 373–395 in Trask (ed.), Recent marine sediments, 1939.
Correns, Carl W. et al. 1937. “Die Sedimente des äquatorialne Atlantischen Ozeans. Deutsche Atlantische Exped” . Meteor1925–1927, Wiss. Erg., Bd. III, 3. Teil, 298 pp., 1937.
Cushman, Joseph A.1928. “Foraminifera: their classification and economic use” . Cushman Lab. Foram. Research, Spec. Publ. no. 1, Sharon, Mass., 401 pp., 1928.
Dietz, R. S., K. O. Emery, and F. P. Shepard. 1942. “Phosphorite deposits on the sea floor off southern California” . Geol. Soc. Amer., Bull., v. 53, p. 815–848, 1942.
Emery, K. O., and Roger Revelle. 1942. “Barite concretions from the ocean floor” . Geol. Soc. Amer. (in press).
Emery, K. O., and R. H. Tschudy. 1941. “Transportation of rock by kelp” . Geol. Soc. Amer., Bull., v. 52, p. 855–862, 1941.
Evans, Robley D., and A. F. Kip. 1938. “The radium content of marine sediments from the East Indies, the Philippines, and Japan, and of the Mesozoic fossil clays of the East Indies” . Amer. Jour. Sci., v. 36, p. 321–336, 1938.
Evans, Robley D., A. F. Kip, and E. G. Moberg. 1938. “The radium and radon content of Pacific Ocean water, life, and sediments” . Amer. Jour. Sci., v. 36, p. 241–259, 1938.
Fleming, Richard H., and Roger Revelle. 1939. Physical processes in the ocean. p. 48–141 in Trask (ed.), Recent marine sediments, 1939.
Fox, Denis L., and L. J. Anderson. 1941. “Pigments from marine muds” . Nat. Acad. Sci., Proc., v. 27, p. 333–336, 1941.
Föyn, Ernst, B. Karlik, H. Pettersson, and E. Rona. 1939. “The radioactivity of seawater” . Oceanografiska Inst. Göteborg (Göteborgs K. Vetensk … Handlingar, 5, Ser. B), Meddelanden, N.S., 44 pp., 1939.
Galliher, E. Wayne. 1933. “The sulfur cycle in sediments” . Jour. Sedimentary Petrol., v. 3, p. 51–63, 1933.
Galliher, E. Wayne. 1935a. “Glauconite genesis” . Geol. Soc. Amer., Bull., v. 46, p. 1351–1366, 1935.
Galliher, E. Wayne. 1935b. “Geology of glauconite” . Amer. Assn. Petrol. Geol., Bull., v. 19, p. 1569–1601, 1935.
Galliher, E. Wayne. 1939. Biotite-glauconite transformation and associated minerals. p. 513–515 in Trask (ed.), Recent marine sediments, 1939.
Goldschmidt, V. M.1933. “Grundlagen der quantitativen Geochemie” . Fortschritte d. Mineral., Kristal., und Petrographie, v. 17, p. 112–156, 1933.
Grim, Ralph E.1939. Properites of clay. p. 466–495 in Trask (ed.), Recent marine sediments, 1939.
Gripenberg, Stina. 1934. “A study of the sediments of the North Baltic and adjoining seas” . Fennia 60, No. 3, 231 pp., Helsingfors, 1934.
Gripenberg, Stina. 1939a. Sediments of the Baltic sea. p. 298–321 in Trask (ed.), Recent marine sediments, 1939.
Gripenberg, Stina. 1939b. Mechanical analysis. p. 532–557 in Trask (ed.), Recent marine sediments, 1939.
Hadding, Assar. 1932. “The pre-quaternary sedimentary rocks of Sweden” . IV. Glauconite and glauconitic rocks. Lunds Geol. Mineralogiska Institution, Medd. Nr. 51, 175 pp., 1932. Lund.
Häntzschel, Walter. 1939. Tidal flat deposits (Wattenschlick). p. 195–206 in Trask (ed.), Recent marine sediments, 1939.
Hewitt, L. F.1937. Oxidation-reduction potentials in bacteriology and biochemistry. 4th ed. London Country Council, no. 3200, 101 pp., 1937.
Hjulström, Filip. 1939. Transportation of detritus by moving water. p. 5–31 in Trask (ed.), Recent marine sediments, 1939.
Hough, J. L.1940. “Sediments of Buzzards Bay, Massachusetts” . Jour. Sedimentary Petrol, v. 10, p. 19–32, 1940.
Johnson, Douglas W.1919. Shore processes and shoreline development. New York, John Wiley and Sons, 584 pp., 1919.
Kelley, W. P.1939. Base exchange in relation to sediments. p. 454–465 in Trask (ed.), Recent marine sediments, 1939.
Kelley, W. P., and G. F. Liebig, Jr.1934. “Base exchange in relation to composition of clay with special reference to effect of sea water” . Amer. Assn. Petrol. Geol., Bull., v. 18, p. 358–367, 1934.
Krumbein, W. C.1939. Graphic presentation and statistical analysis of sedimentary data. p. 558–591 in Trask (ed.), Recent marine sediments, 1939.
Krumbein, W. C., and F. J. Pettijohn. 1938. Manual of sedimentary petrography. New York, D. Appleton-Century Co., 549 pp., 1938.
Kuenen, Ph. H.1937. “On the total amount of sedimentation in the deep sea” . Amer. Jour. Sci., v. 34, p. 457–468, 1937.
Kuenen, Ph. H.1941. “Geochemical calculations concerning the total mass of sediments in the earth” . Amer. Jour. Sci., v. 39, p. 161–190, 1941.
Martens, James H. C.1939. Beaches. p. 207–218 in Trask (ed.), Recent marine sediments, 1939.
Mehmel, Martin. 1939. Application of X-ray methods to the investigation of recent sediments. p. 616–630 in Trask (ed.), Recent marine sediments, 1939.
Moore, Hilary B.1931. “The muds of the Clyde Sea area. III. Chemical and physical conditions; rate and nature of sedimentation; and fauna” . Marine Biol. Assn. U. K., Jour., v. 17, p. 325–358, 1931.
Moore, Hilary B.1939. Faecal pellets in relation to marine deposits. p. 516–524 in Trask (ed.), Recent marine sediments, 1939.
Murata, K. J.1939. “Exchangeable manganese in river and ocean muds” . Amer. Jour. Sci., v. 37, p. 725–735, 1939.
Murray, Sir John, and James Chumley. 1924. “The deep-sea deposits of the Atlantic Ocean” . Royal Soc. Edinburgh, Trans., v. 54, pt. 1, 252 pp., 1924.
Murray, Sir John, and J. Hjort. 1912. The depths of the ocean. London, Macmillan, 821 pp., 1912.
Murray, Sir John, and E. Philippi. 1908. “Die Grundproben der Deutschen Tiefsee Expedition” . Deutsche Tiefsee-Exped. Valdivia 1898–1899, Wiss. Erg., Bd. 10, p. 77–206, 1908.
Murray, John, and A. F. Renard. 1891. “Report on deep-sea deposits based on the specimens collected during the voyage of H.M.S. Challenger in the years 1872 to 1876” . Challenger Repts., 525 pp., 1891.
Neaverson, E.1934. “The sea-floor deposits. I. General characteristics and distribution” . Discovery Repts., v. 9, p. 297–349, 1934.
Pia, Julius, 1933. “Die rezenten Kalksteine” . Zeitschr. f. Kristallographie, Mineral. u. Petrogr., Abt. B, Ergänzungsband, 420 pp., 1933. Leipzig.
Prandtl, L.1932. “Meteorologische Anwendungen der Strömungslehre” . Beiträge z. Physik d. freien Atmosphäre, Bd. 19, Leipzig, 1932.
Pratje, Otto. 1939a. “Sediments of South Atlantic Ocean” . Amer. Assn. Petrol. Geol., Bull., v. 23, p. 1666–1672, 1939.
Pratje, Otto. 1939b. “Die Sedimente des Südatlantischen Ozeans” . 2. Lief. Die Untersuchungsergebnisse nach Stationen geordnet. Deutsche Atlantische Exped.Meteor1925–1927, Wiss. Erg., Bd. III, 2. Teil, 2. Lief., p. 57–171, 1939.
Radczewski, O. E.1939. Eolian deposits in marine sediments. p. 496–502 in Trask (ed.), Recent marine sediments, 1939.
Revelle, Roger. 1936. “Marine bottom samples collected in the Pacific Ocean by the Carnegie on its seventh cruise” . Dissertation submitted in partial satisfaction of the requirements for the degree of doctor of philosophy in oceanography, Univ. of Calif., 317 ms. pp., 1936. (In Press, Carnegie Inst. Washington, Dept. Terr. Magnetism.)
Revelle, Roger, and F. P. Shepard. 1939. Sediments off the California coast. p. 245–282 in Trask (ed.), Recent marine sediments, 1939.
Ridgway, Robert. 1912. Color standards and color nomenclature. Washington, D. C., publ'd by the author, 43 pp., 53 colored pls., 1912.
Rouse, Hunter. 1938. Fluid mechanics for hydraulic engineers. New York, McGraw-Hill Book Co., 422 pp., 1938.
Russell, R. Dana. 1939. Effects of transportation on sedimentary particles. p. 32–47 in Trask (ed.), Recent marine sediments, 1939.
Russell, Richard J., and R. Dana Russell. 1939. Mississippi river delta sedimentation. p. 153–177 in Trask (ed., Recent marine sediments, 1939.
Schalk, Marshall. 1938. “A textural study of the outer beach of Cape Cod, Massachusetts” . Jour. Sedimentary Petrol., v. 8, p. 41–54, 1938.
Schott, Gerhard. 1935. “Geographie des Indischen und Stillen Ozeans” . Deutsche Seewarte, Hamburg, 413 pp., 1935.
Schott, Wolfgang. 1937. Die Foraminiferen in dem äquatorialen Teil des Atlantischen Ozeans. p. 43–134 in Correns et al, 1937, which see above.
Schott, Wolfgang. 1939a. Deep-sea sediments of the Indian Ocean. p. 396–408 in Trask (ed.), Recent marine sediments, 1939.
Schott, Wolfgang. 1939b. Rate of sedimentation of recent deep-sea sediments. p. 409–415 in Trask (ed.), Recent marine sediments, 1939.
Schuchert, Charles. 1931. “Geochronology, or The age of the earth on the basis of sediments and life” . p. 10–64 in Physics of the earth, v. 4, Age of the earth. Nat. Research Council, Bull.no. 80, 1931.
Shepard, Francis P., and E. C. La Fond. 1940. “Sand movements along the Scripps Institution pier” . Amer. Jour. Sci., v. 38, p. 272–285, 1940.
Shepard, Francis P., and G. A. MacDonald. 1938. “Sediments of Santa Monica Bay, California” . Amer. Assn. Petrol. Geol., v. 22, p. 201–216, 1938.
Shepard, Francis P., K. O. Emery, and E. C. La Fond. 1941. “Rip currents: a process of geological importance” . Jour. Geol., v. 49, p. 337–369, 1941.
Smith, C. L.1940. “The Great Bahama Bank. II. Calcium carbonate precipitation” . Jour. Marine Research, v. 3, p. 171–189, 1940.
Stetson, Henry C.1938. The sediments of the continental shelf off the eastern coast of the United States. Papers in Physical Oceangr. and Meteorol., v. 4, no. 4, 48 pp., 1938.
Stetson, Henry C.1939. Summary of sedimentary conditions on the continental shelf off the east coast of the United States. p. 230–244 in Trask (ed.), Recent marine sediments, 1939.
Ström, Kaare M.1936. “Landlocked waters. Hydrography and bottom deposits in badly-ventilated Norwegian fjords with remarks upon sedimentation under anaërobic conditions” . Norske Vidensk. Akad. i Oslo, Skirfter, I. Mat.-Naturv. Klasse, no. 7, 85 pp., 1936.
Ström, Kaare M.1939. Landlocked waters and the deposition of black muds. p. 356–372 in Trask (ed.), Recent marine sediments, 1939.
Sverdrup, H. U., and Staff. 1941. “Research in physical oceanography and submarine geology at the Scripps Institution of Oceanography during April, 1940, to April, 1941” . Amer. Geophys. Un., Trans., p. 490–494, 1941.
Takahashi, Jun-ichi. 1939. Synopsis of glauconitization. p. 503–512 in Trask (ed.), Recent marine sediments, 1939.
Thompson, Warren O.1937. “Original structures of beaches, bars, and dunes” . Geol. Soc. Amer., Bull., v. 48, p. 723–752, 1937.
Throp, Eldon M.1931. “Descriptions of deep-sea bottom samples from the western North Atlantic and the Caribbean Sea” . Scripps Inst. Oceanogr., Bull., v. 3, p. 1–31, 1931.
Trask, Parker D.1932. “Origin and environment of source sediments of petroleum” . Houston, Amer. Petrol. Inst., Gulf Publ. Co., 323 pp., 1932.
Trask, Parker D.1937. “Relation of salinity to the calcium carbonate content of marine sediments” . U. S. Geol. Survey, Prof. Paper 186, p. 273–299, 1937.
Trask, Parker D.1939. Organic content of recent marine sediments. p. 428–453 in Trask (ed.), Recent marine sediments, 1939.
Trask, Parker D. (ed.)1939. “Recent marine sediments” . Tulsa, Amer. Assn. Petrol. Geol., 736 pp., 1939.
Twenhofel, William H.1926. “Treatise on sedimentation” . Baltimore, Williams and Wilkins Co., 661 pp., 1926.
Twenhofel, William H.1932. Treatise on sedimentation. 2nd ed., completely revised. Baltimore, Williams and Wilkins Co., 926 pp., 1932.
Twenhofel, William H.1939. Principles of sedimentation. New York, McGraw-Hill Book Co., 610 pp., 1939.
U. S. Beach Erosion Board. 1933. Interim report. Washington, D. C., U. S. Army, Office of Chief of Engineers, mimeographed, 1933.
Urry, W. D., and C. S. Piggot. 1942. “Radioactivity of ocean sediments. V. Concentration of the radio-elements and their significance in red clay” . Amer. Jour. Sci., v. 240, p. 93–103, 1942.
Utterback, C. L., and L. A. Sanderman. 1938. “Radium content of some inshore bottom samples in the Pacific Northwest” . Jour. Marine Research, v. 1, p. 187–191, 1938.
Vaughan, T. Wayland. 1924. “Oceanography in its relation to other earth sciences” . Washington Acad. Sci., v. 14, p. 307–333, 1924.
Washington, Henry S.1920. “The chemistry of the earth's crust” . Franklin Inst., Jour., v. 190, p. 757–815, 1920.
Wiseman, John D. H., and H. Bennett. 1940. “The distribution of organic carbon and nitrogen in sediments from the Arabian Sea” . John Murray Exped. 1933–34, Sci. Results, v. 3, p. 193–221, 1940.
ZoBell, Claude E.n 1939. Occurrence and activity of bacteria in marine sediments. p. 416–427 in Trask (ed.), Recent marine sediments, 1939.
ZoBell, Claude E., and D. Q. Anderson. 1936. “Vertical distribution of bacteria in marine sediments” . Amer. Assn. Petrol. Geol., Bull., v. 20, p. 258–269, 1936.