10. RACE AND HEALTH
Implications for Health Care
Delivery and Wellness Promotion
Mack Roach III
INTRODUCTION: RACE AND HEALTH
“Race” has been defined as “a subdivision of the human species, characterized by a more or less distinctive combination of physical traits that are transmitted in descent” and “health” as “the general condition of the body or mind with reference to soundness and vigor.” 1 There is no obvious reason for these “concepts” to be linked. However, large differences have been noted in the state of “health” between the various racial groups for many years. Racism and the lack of quality of health care are not unique to African Americans, but because the author is most familiar with their impact on this group, the discussion to follow will emphasize these issues as they relate to them. The author believes that this discussion addresses concerns that are universal and should be of value to all Americans who are forced to live with people with skin of a different color than their own and/or to vote on issues related in any way to race and ethnicity.
African Americans have always had a lower survival rate than Whites in this country. Evidence has also always existed suggesting that “nongenetic” explanations have been the dominant causes. For example, based on a study conducted in 1908, Irish and Italian men living in New York City actually had a higher mortality rate than Black men living at that time.2 Furthermore, for many years it has been known that discrepancies in survival within racial groups varied more by residence (rural vs. urban) than between races.3 It is also noteworthy that recent
Despite the kinds of data noted here, a body of literature has been perpetuated for many years in this country arguing that “race” is a biologic phenomenon associated with some less-than-desirable health consequences. James H. Jones summarized the position of prominent 19th-century physicians in his book Bad Blood:5,6
Vociferous advocates of black inferiority such as Dr. Josiah Clark Nott of Mobile and Dr. Samuel A. Cartwright of New Orleans published numerous articles during the 1840s and 1850s on diseases and physical properties thought to be peculiar to blacks. Drs. Nott and Cartwright were merely the best known of a group of southern physicians who helped inflame the controversy over slavery. Among the diseases said to be unique to blacks were Cachexia Africana (dirt-eating) and Struma Africana (“Negro consumption”). Influenced by these physicians, slave holders who wished to treat their bondsmen without benefit of professional help begged southern doctors to write medical manuals on the treatment of blacks. Their requests went unanswered. Instead, physicians simply continued to assert that blacks were medically inferior to whites without offering a plausible medical explanation based on racial differences. Their observations were perfect for polemics but useless for the care of sick blacks.
It was in fact this type of thinking that led to the Tuskegee syphilis experiments. Researchers from the U.S. Public Health Service in the 1930s justified studying untreated syphilis in Blacks because they believed that they knew the natural history of syphilis in Whites (based on an old Scandinavian study) and wanted to prove the hypothesis that syphilis was different in Blacks. Since this atrocity, numerous other papers have been published that imply that being a member of the Black race has a detri-mental/adverse effect on the length of survival. Implicit in many of these studies is the suggestion that race is a “real biologic factor,” meaning that it must be considered as a separate factor from tumor- or treatmentrelated factors.
For more than 20 years there has existed clear documentation of an excess cancer mortality in Black Americans compared to White Americans.5 This excess mortality experienced by Black Americans is associated with a disproportionate financial burden because of the lower incomes, higher unemployment, and the inadequacy of health care resources that are currently available to this community. The impact of the excess mortality rate and the financial burden are magnified by the increased incidence of common cancer sites among Black Americans.
The assumption that there are major differences in biologic behavior related to race continues to be a theme of medical doctors even today. For example, in the July 1997 issue of the prestigious peer-reviewed Journal of Urology, Moul et al. published a paper titled “Black Race Is an Adverse Prognostic Factor. …” 7 Later, in an even more widely read journal, Cancer, these investigators published an article describing an equation that could be used to predict the risk of failing a radical prostatectomy that incorporated “Black race” as an unfavorable biologic parameter.8 Both of these papers included relatively small numbers of patients, and neither provided an in-depth discussion of alternative explanations. The successful publication of these papers, despite their failure to discuss other explanations, suggests that the reviewers were in agreement with the authors. Clearly there appears to be a critical mass of researchers who believe that “race” has an intrinsic impact on health. As a result of this established dogma, some researchers have found it difficult to publish papers opposing this notion.
For example, in the 1980s a paper was published in Cancer describing the poor outcome of Blacks (n = 92) treated for laryngeal cancer at Harlem Hospital. In response to this paper, I submitted a paper representing a 20-year experience from a Veterans Administration hospital including more than 300 patients demonstrating that the long-term survival in Blacks and Whites was identical.2 In our paper, I explained that based on the details provided, the care delivered appeared to have been suboptimal in the previously published paper. A major criticism of our paper (resulting in a rejection by Cancer) was that we would need “1200 patients to prove that Blacks did not do worse.” Since there has never been a paper published in the world's literature on laryngeal cancer that included more than approximately 600 patients, we were placed into an obvious “catch-22” situation. The reviewers believed that the burden of proof should lay on our shoulders and that, until proven otherwise, race should be considered a significant independent determinant of outcome. But is this true?
The answer to this question has a number of implications for the delivery of health care. First, the recognition of genetic differences could allow specific populations to be targeted for the delivery of certain types
DO WE REALLY WANT TO KNOW?
Before discussing data assessing the merits of genetic and nongenetic causes, there are several questions that should be answered. First, since race has “been around forever,” why do these questions still persist? Is this due to lack of data, or could it be that “we” really do not want to know? Stephen J. Gould recognized this issue and began his book The Mismeasure of Man with the following quote from Charles Darwin's Voyage of the Beagle:9
If the misery of our poor be caused not by the laws of nature, but by our institutions, great is our sin.
If the basis for the excess mortality among certain racial groups is an intrinsic characteristic of the group, some might consider this a sign of “racial inferiority.” Although such a possibility would say nothing about the moral, creative, humanitarian, or other more important features of an individual, members of such a racial group are still likely to be defensive. Conversely, if the excess mortality rate is entirely due to various types of social injustices (such as racism and discrimination, resulting in lack of education, underemployment, and poor access to care, resulting in a fatalistic self-destructive lifestyle), the moral and financial implications would be staggering.
To understand the development of the notion of some sort of inherent tendency for Blacks to be “genetically less healthy,” it is best to assess the sources of this belief. Therefore, it is important to look back at the history of events in the history of this country that might have had
RACE AND HEALTH IN A HISTORICAL CONTEXT:
MOSTLY A BLACK-AND-WHITE ISSUE?
Just as the issue of “racism in America” is usually perceived as largely a “Black versus White” issue, the issue of “race and health” is often seen in a similar context. For example, annual reports sponsored by the federal government compare outcomes between Blacks and Whites and routinely ignore other groups.5 It is a simple matter to find 20 to 30 publications comparing outcomes between Black women and White women with breast cancer, but similar studies for other groups are lacking. This reality may result from the several facts. First, Hispanics are generally considered as an ethnic group, not as a race. When Hispanics are placed into one of the major three groups (Blacks, Whites, and Asians), they are for the most part considered “White.” Second, for Asians, including Pacific Islanders, and Native American Indians, the details surrounding the impact of racism on their health is both complex and heterogeneous. This is not to suggest that the health issues for these groups are any less important but rather that (1) they are not as well documented, (2) for most health outcome end points (e.g., death due to cancer) the differences are not as large and in some cases favor the Asian populations,
(3) differences in social status have not been as clearly enforced by the laws of the land, and (4) over the last 400 years fewer individuals belonging to this “racial group” have been impacted by racism.
The circumstances surrounding the arrival of African people to this country are likely to explain some of the problems in health status seen today. Between 1501 and 1870, it has been estimated that between 9.5 and 14.6 million African people were brought to America in bondage.10 Furthermore, it is believed that nearly as many African people died, resisting capture, via suicide, or en route, due to hardships. The mortality rates at sea alone have ranged from as high as 33% to as low 12%.11 It is obvious that the first generation of African Americans had a very short life expectancy. Harley has summarized selected historical events reflecting social events of note in the history of African Americans, and some of these are listed in Table 10.1.12 Harsh punishments and intolerance were the rule, and it was more than 250 years before the first Black man (who was a slave at the time) was licensed as a physician. This being the
| Years | Event | Comments |
|---|---|---|
| 1492 | Pedro Alonzo Nino, a navigator of the Santa Maria, arrives with Christopher Columbus. | |
| 1502 | Portugal lands its first cargo of enslaved Africans in the Western Hemisphere. | |
| 1501 to 1870 (369 years) | Slavery delivered between 9.5 and 14.6 million African people to the Americas, and nearly as many are thought to have died, resisting capture, via suicide, or en route due to hardships. | After 369 years of slavery, and other acts of violence, what reparations would be due these people and their offspring? |
| 1692 | Virginia enacts law making it lawful to kill a runaway slave in the course of apprehension. | |
| 1693 | Philadelphia: Law permitting Whites to “take up” any Black found without a pass. | |
| 1762 | James Derham becomes the first Black man licensed to practice medicine in the United States and 21 years later purchases his freedom. | |
| 1809 | New York law sanctions marriage within the Black community. | Married Blacks were not legally recognized as such before this law. |
| 1810 | 19% of the U.S. population is Black, but only 9% are free. | |
| 1863 | The Emancipation Proclamation goes into effect, freeing slaves held in states in rebellion against the Union, but not in portions of Louisiana, Eastern Virginia, West Virginia, or border states (3). | Although slaves were freed, they were not able to vote and continued to be systematically oppressed. |
| 1866 | The first Civil Rights Act is passed over President Andrew Johnson's veto, declaring Blacks free and nullifying “Black codes.” | “Black codes” restrict the rights of freedmen/women. |
| 1868 | 14th Amendment is passed, granting Blacks “full citizenship and equal rights.” | |
| 1890 | U.S. Supreme Court allows states to segregate public facilities and control of elections. | |
| 1896 | “Separate but equal” facilities ruled constitutional. | |
― ―
1898-1910 | Louisiana, Georgia, North Carolina, Virginia, Alabama, and Oklahoma adopt the “grandfather clause.” | Males could vote only if their fathers or grandfathers were eligible to vote. |
| 1932 | Tuskegee experiments begun. | |
| 1940 | U.S. Congress passes Selective Training and Service Act. | Includes an antidiscrimination clause and a 10% quota system to ensure racial integration. |
| 1957 | Civil Rights Act of 1957 passed, authorizing the federal government to bring civil suits on the behalf of citizens. | First Civil Rights Act since 1875. |
| 1964 | U.S. Congress passes the Civil Rights Act and establishes the Equal Opportunity Commission (EEOC). | This law was passed to offset hundreds of years of systematic discrimination and racism, but it failed. |
| 1971 | National Cancer Act and SEER program established. | SEER created to collect, analyze, and disseminate data. |
| 1994 | Black-White Breast Cancer Study: Race not an independent prognostic factor. | Having a high poverty index, lack of insurance, and increased body mass index, and being divorced, separated, or never married associated with a poor outcome. |
| 1994 | RTOG 9202 demonstrates that Blacks have more advanced prostate cancer. | More advanced prostate cancer by virtue of higher PSAs not in the clinical stage. |
| 1995 | Proposition 209 goes into effect in California, banning Affirmative Action to compensate for past discrimination. | How many years of affirmative action compensate for 369 years of slavery and many years of systematic discrimination and oppression? |
| 1996 | RTOG 9412 and the A2 demographic studies. | Both demonstrate that Blacks continue to have lower incomes, less education, and more advanced disease. |
| 1997 | CALGB 8541, based on a prospective randomized trial including 1,500 women; race not an independent factor. | Black women continue to have an excess mortality rate from breast cancer. |
| 1997 | CA Journal published demonstrating 59% five-year survival for Whites versus 44% for Blacks. | This 1.4-times greater risk of cancer death is the largest recorded since 1960. |
| 1997 | Courts rule against “set-aside programs” in Philadelphia for city works programs. | Similar programs struck down in Columbus, Ohio, and Miami. |
Of note, although the Emancipation Proclamation was passed in 1863 freeing some slaves, it did not apply in portions of Louisiana, Eastern Virginia, West Virginia, or border states. Moreover, freed men and women continued to be systematically oppressed. Consequently, three years after the Emancipation Proclamation, “Black codes” (which systematically restricted the rights of freed men and women) were passed over the veto of the U.S. president.
Two years later the 14th Amendment granted African Americans full citizenship and equal rights. However, 30 years later “separate but equal” was ruled constitutional and “grandfather clauses” (allowing males to vote only if one's father or grandfather voted) were upheld in a number of states (1898–1910). Finally, after 369 years of slavery and 87 years of systematic discrimination, the Civil Rights Act of 1957 was passed. Later the U.S. Congress passed the Civil Rights Act of 1964.
In 1971 the National Cancer Act establishing the SEER (Surveillance Epidemiology End Result) program was created to collect, analyze, and disseminate data useful in the diagnosis and treatment of cancer.1 These SEER data are published annually and are considered to be the “gold standard” by physicians throughout this country. From 1973 to 1990 information on approximately 1.6 million cases has been collected. Approximately 9.6 percent of the population of the United States is included in the geographic areas making up the database for the SEER program.1 Since the natural histories of treated and untreated cancers of various types have been well studied, this disease will be considered in some detail to assess the prognostic significance of race.
RACE, CANCER SURVIVAL, AND SEER DATA
A close look at the primary cancer sites for which differences between White and Black Americans are most apparent is required to identify the causes for the survival discrepancies. Five-year survivals based on SEER data for all cancer sites among Blacks and Whites is shown in Figure 10.1. Although the five-year relative survival rate is 54.5% for White patients, it is only 39.4% for Blacks.1 These data suggest that if you are Black and diagnosed as having cancer, your risk of dying from cancer within five years is 50% higher than for Whites. Equally alarming is the fact that the percentage change in the mortality from 1973 to 1990
Figure 10.2 compares the outcome for Black and White men with carcinoma of the prostate. Only 64.4% of Black men diagnosed with prostate cancer were alive at five years compared to 79.4% of White men. This marked difference in survival appears to be a continuing trend with a greater increase in the mortality in Blacks compared to Whites from 1973 to 1990. Of further interest, a much greater increase in the percentage change in incidence was noted among Whites. This trend probably reflects the more frequent use of the serum marker PSA (prostatespecific antigen) to detect otherwise occult disease in this population. In other words, although the risk of prostate cancer is lower among White men than among Black men, more of the former are systematically being screened.
Figure 10.3 compares the outcome for breast cancer by race. Again a lower survival is noted for Black women compared to White women, with 64.2% and 80.5%, respectively, alive at five years. A 21.4% increase in the percentage change in mortality was noted for Blacks compared to 2% for Whites during this same time period (1973–1990).
The tendency for Black Americans to present with more advanced disease is one of the common explanations offered for these difference in survival.13 However, even after correcting for the stage of disease, many studies still report an excess mortality rate among Blacks.1, 13–15 For selected sites, differences in socioeconomic status (SES) have also been proposed as an explanation for differences in survival.14,16,17 However, some studies failed to demonstrate an effect due to SES when the quality of care was comparable.18,19 Furthermore, a biologic mechanism explaining how SES affects outcome is lacking. The possibility that differences in cancer-related mortality might be due to factors such as the quality of the medical care received has not been adequately evaluated. Several studies document differences in initial treatment, patterns of care, the intensity of services provided, as well as a tendency for racial bias in the inpatient setting.20–24 These last two observations support the notion that lifestyle and nongenetic factors may be the overwhelming determinant of the health status for most people. The fact that recent studies continue to demonstrate changes in mortality in both races as a reflection of lifestyle changes provides additional support for the truism that “you are what
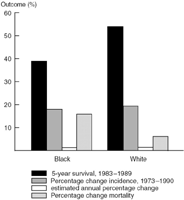
Figure 10.1. SEER race data, all sites.
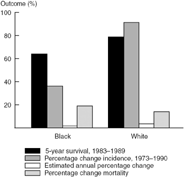
Figure 10.2. SEER race data, prostate cancer.
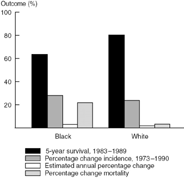
Figure 10.3. SEER race data, breast cancer.
RACE AND HEALTH CARE POLICIES
AND THE CURRENT BELIEF CONSTRUCT
It is clear that Blacks have a lower survival than Whites for a number of common cancers. In response to these kinds of data, in late 1993 the National Cancer Institute (NCI) mandated that cooperative groups conducting large prospective randomized trials involving cancer treatment must include in their study design mechanisms to address the issue of race and cancer outcome (if the published literature suggests that race or gender might affect outcome).25 This mandate represents a major health care policy decision that could potentially impact the design and implementation of most randomized trials conducted in the United States because for most of the common cancer sites there is a discrepancy in survival between Blacks and Whites.
In addition to explicitly affecting cancer research protocol design policies, the published literature supporting the intrinsic importance of
| Conclusions | Results |
|---|---|
| Cancer survival differences exist for Blacks and Whites. | Race is assumed to be an independent prognostic factor. More data are generated to prove that differences exist. |
| The burden of proof to the contrary rests on others attempting to disprove. | Biologic basis is assumed for the observed differences in survival by race. |
| This “biologic” phenomenon should be studied further. | Cancer researchers are funded to study the “racial biology.” |
| There is nothing you can do about a person's race but … | … perhaps understanding the molecular basis of cancer wilt be directly helpful (to researchers). |
This mind-set is contrary to what should be acknowledged as our social norm. Consistent with our social norms opposing racial stereotypes, race should be implicated only as a diagnoses by exclusion. In other words, only after other plausible explanations have been ruled out should race be implicated. If the survival differences noted were attributed to differences in the stage at presentation or to the quality of cancer care received, race should not be the focus of investigation.
AN ALTERNATIVE BELIEF
CONSTRUCT AND EXTENT OF DISEASE BIAS
An alternative belief construct to explain the observed differences in survival by race is summarized in Table 10.3. The implications for this alternative
| Beliefs | Implications |
|---|---|
| Cancer survival differences between Blacks and Whites can be explained by differences in the extent of disease at diagnosis and quality of care. | Race is not an independent prognostic factor. |
| The burden of proof to the contrary rests on others attempting to prove that race is a prognostic factor. | “Racism” is not perpetuated by “science.” |
| Differences in outcomes should be studied further to identify causes. | Research should focus on differences in the knowledge, behavior, and environment as well as on access and the quality of care. |
| There is something that you can do about lack of jobs, education, environmental factors, and quality of health care. Lack of health is a symptom of social diseases. | Intervention is directly helpful to the people being studied and experiencing the excess mortality. Social changes improve health. |
An epidemiologic phenomenon that I have chosen to call “extent of disease bias” (EDB) may explain much of the reported survival differences. Staging systems represent somewhat arbitrary ways to separate patients in prognostic groups for the purposes of comparison.26,27 Historically, these systems usually were primarily based on the size of the tumor as determined by palpation and usually referred to as the “T” stage, with categories 1 through 4.27 These systems typically also depend on the extent of lymph node involvement, defined by size, location, and number.27–30 The break points for these staging systems typically reflected whether it was believed that a tumor could be completely resected.
Extent of disease bias results from two major types of shortcomings of the current staging systems. First, the currently used staging systems are overly crude in the degree of absolute separation or “the degree of fineness of separation” of distinct prognostic groups. Second, the current staging systems are designed primarily to answer a question of the relative probability of cure rather than the duration of survival among those patients who are not curable. This shortcoming reflects “the disproportionate predictive priorities” of the current staging systems.
THE EXTENT OF DISEASE BIAS (EDB) MODEL
Figures 10.4 to 10.6 compare two hypothetical populations with differences in the distribution of the extent of disease at presentation. Population A is composed of a cohort of relatively well educated individuals, of higher socioeconomic status, who tend to present with earlier-stage disease. Cohort B is composed of individuals of lower socioeconomic status, with less education and a high percentage being uninsured, who tend to present with more advanced disease. In this model it is assumed that for each population of cancer patients there is a “bell-shaped” distribution of disease-specific survivals that correlates with the extent of disease. This disease-specific survival distribution is a direct reflection of, and consequently is proportional to, the extent of disease. The term “extent of disease” as used in this model takes into account the volume of the tumor and the degree to which the tumor has spread. Extent of disease also includes the fact that with time, biologic changes tend to occur in the aggressiveness of tumors, which may not be manifested as differences in the tumor volume or extent of spread.
Figure 10.4 compares the distribution of cancer in populations A and B using a relatively “crude” staging system, with early, intermediate, and advanced disease corresponding to stages 1, 2, and 3, respectively. Of note, a larger percentage of individuals in population B have stage 3 disease and fewer have stage 1, while a similar percentage of members from both populations have stage 2 disease. It would seem appropriate to most observers that a survival comparison of patients with stage 2 disease from populations A and B would be valid. This may not be the case, however.
Figure 10.5 compares the same groups using a “superstaging” system composed of 12 stage categories instead of three. Figure 10.6 is a “magnified view” of the intermediate extent of disease group. This intermediate extent of disease group corresponds to superstages 5 to 8 and the “crude” stage 2 group. Using the superstaging system it is now apparent that more group B members belonging to the intermediate extent of disease group have superstage 8 and that more group A members have superstage 5. Because of these differences, group B members would be expected to do worse than group A members. An analysis using crude staging would suggest that group membership had independent prognostic significance. In contrast, an analysis using the superstaging system is likely to demonstrate that group membership has no prognostic significance independent of the true extent of disease (see Figures 10.5 and 10.6).
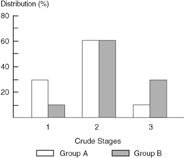
Figure 10.4. Extent of Disease Bias Model: Example of distribution differences in two populations of patients with cancer.
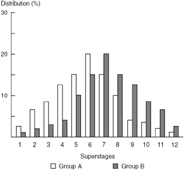
Figure 10.5. Extent of Disease Bias Model: Comparing distribution differences in two populations with cancer using superstaging.
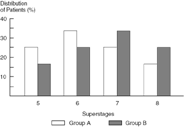
Figure 10.6. Extent of Disease Bias Model Example: Superstages 5 to 8 versus crude stage 2.
SEER DATA AND THE EDB MODEL
Examples taken from published literature demonstrating the potential for EDB for Blacks and Whites with prostate and breast cancer are shown in Figures 10.7 and 10.8. Note that in both the examples shown the distribution of disease is similar to that of populations A and B in the EDB crude staging model. The extent of disease in cancer patients is not naturally divided into three or four distinct groups. Rather, there is a continuum. The less precisely populations are defined, the more likely patients with extensive disease will be “lumped” with patients with less extensive disease. Therefore, differences in the distribution characteristics in two populations can confound an analysis of outcome.
Examples of the Potential for EDB from
(Non-SEER-Based) Cancer Treatment Literature
In addition to SEER data, numerous other examples of distribution differences between Blacks and Whites exist in the medical literature.13,14,26 The same pattern is seen in all these studies: The distribution of cancer in Blacks is “right shifted.” The term “right shifted” refers to the relative displacement of the overall shape of the extent of disease distribution toward more advanced stages for Blacks compared to less advanced disease for Whites. The “cruder” the staging system used, the more biased the interpretation is likely to be.
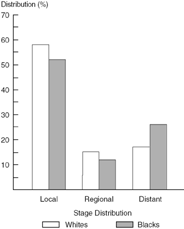
Figure 10.7. SEER prostate cancer: Stage distribution data, 1983 to 1987.
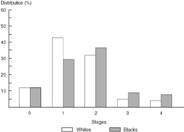
Figure 10.8 SEER breast cancer: Stage distribution data, 1990.
Examples of how the degree of fineness of separation within a given stage can affect prognosis are available throughout the cancer literature. Rosen et al., for example, reported that within the category of patients with T1 NoMobreast carcinoma (stage I), significant differences in prognosis exist based on the size of the primary tumor.28 For example, patients with a primary breast tumor less than 1.0 centimeter had an 83% relapse free survival (RFS) at 10 years compared to a 73% RFS for patients with primaries 1.1 to 2.0 centimeters. This represents an excess relapse risk of 59% at 10 years. Differences of this magnitude are common in the series, which report breast cancer survival differences between Blacks and Whites. If the distribution of the extent of breast cancer was such that a larger percentage of Black women had lesions in the 1.1- to 2.0-centimeter range and a larger percentage of Whites had lesions in the range less than or equal to 1.0 centimeter, it is possible that despite “correcting for stage,” an analysis might suggest that there were survival differences due to race.
DISPROPORTIONATE PREDICTIVE PRIORITIES
The current staging systems are designed primarily to predict surgical curability.27–30 Patients who are not curable tend to be lumped into a category that is composed of a heterogeneous group of individuals with a poor long-term prognosis. Although such patients uniformly have a poor long-term prognosis, there can be significant differences in shortterm survival. These differences can significantly affect the overall survival curve because a large number of patients fall into this category. Examples can be found in the literature where the differences in survival for patients belonging to the same stage may vary from less than 6 weeks to 6 months to a few years.29,30 Thus, the current staging systems have disproportionate predictive priorities in the sense that with an increase in the extent of disease, the staging system becomes progressively less discriminating in terms of identifying various prognostic subgroups.
An example of these phenomena is the ability of the current staging systems used to predict the outcome for the average patients with lung cancer. Although most patients who present with lung cancer do not have curable disease (overall five-year survival is about 10%), the staging system is biased in favor of subcategories of patients who are considered potentially surgically resectable.30 The most widely used staging system is characterized by having four out of six of the major substaging categories dedicated to the small percentage of patients who are considered
BREAST CANCER AND EDB:
RETROSPECTIVE STUDIES
Although SEER data and numerous studies report a lower survival for Black women with breast cancer, several recent studies strongly suggest that race is not an independent prognostic factor.13,14,16,18,26,31–42 A detailed analysis of this issue was reported by Roach and Alexander.26 These authors reviewed the available published literature comparing the survival of Blacks to Whites with breast cancer treated from 1968 to 1988. The five-year survival for Blacks was less than or equal to that of Whites in all studies (difference range about 0 to 19%). However, in 11 of the 18 studies (61%), the reported differences in five-year survival between Blacks and Whites was less than or equal to 3%. We generated a “reliability scoring scale” based primarily on the level of staging sophistication used (crude staging resulted in lower scores) and the likelihood that the quality of care was comparable. Next the relationship between the reliability score and survival was analyzed. Those studies reporting large differences in five-year survival generally had low reliability scores, while those with high scores tended to have small differences in survival.
BREAST CANCER DATA FROM
PROSPECTIVE RANDOMIZED TRIALS
Using patients treated on phase III prospective randomized trials avoids potential bias due to differences in the initial staging workup and treatment;
The findings of these studies are consistent with the EDB model. However, there are some differences in the presentation of breast cancer in Black and White women that remain unexplained. For example, it is well known that breast cancer is less common in Black women, and some studies suggest that there is an earlier age of onset.44–46 These differences may be due to the average age of first pregnancy, diet, intrinsic genetic differences, or other factors yet to be defined. Table 10.4 summarizes the findings reached following a prospective study of Black and White women with breast cancer that demonstrated that poverty, martial status, health insurance status, and body mass but not race correlated with death due to breast cancer.46 Failure to account for differences in these areas may explain at least a portion of the impression reached by some researchers that race was likely to be an independent prognostic factor.
PROSTATE CANCER AND EDB:
RETROSPECTIVE STUDIES
A number of retrospective studies have been published addressing the prognostic significance of race and survival from prostate cancer.12,13,15,17,18,23,47–53 Austin et al. conducted a retrospective study including 914 patients (867 Whites, 47 Blacks) treated with radiation
| Factor | Relative Risk[a] |
|---|---|
aUndajusted hazard ratios (95% confidance intervals), modified from Eley et al. (1994 [46]). | |
| Poverty index > 400 (high income) | 0.6 × (0.4-0.7) |
| Divorced, separated | 1.6 × (1.2-2.2) |
| Never married | 1.6 × (1.0-2.5) |
| No health insurance | 2.3 × (1.5-3.5) |
| High body mass index (“overweight”) | 2.2 × (1.5-3.2) |
A number of other investigators have reported race to be of no independent significance after adjustment for other prognostic factors.51–53 In contrast to the series reporting a difference in survival as a function of race, either all these series were likely to have provided a similar quality of care (single institution study or as part of a randomized trial) or an adjustment was made for socioeconomic status (perhaps a predictor for quality care). This is important because of studies showing that Blacks tend to be treated less aggressively.18
DATA FROM PROSPECTIVE
RANDOMIZED TRIALS: PROSTATE CANCER
The largest prospective database available for assessing the outcome following definitive radiotherapy for clinically localized prostate cancer is possessed by the Radiation Therapy Oncology Group (RTOG). Using
Based on the available literature, the preponderance of evidence suggests that after adjustment for the extent of disease, when treatment is comparable, there are no differences in survival from early stage prostate cancer that can be attributed to race.51–54 For more advanced disease, it is likely that the survival differences reflect differences in the distribution of disease at the time of diagnoses that are not accounted for in the staging system used.54,55 Since many of the patients treated for prostate cancer are staged clinically and not pathologically, accurately determining the true extent of disease in prostate cancer patients is more difficult than for breast cancer. These inaccuracies in staging, and the differences in the disease distribution in these two populations, result in EDB. Additionally, there are a multitude of socioeconomic differences that might explain differences in outcome.56 For example, in a large demographic study we noted clear differences in income status and the educational level of Blacks and Whites treated on phase III randomized trials. Clearly, having less support and a poorer understanding of your disease is not likely to be beneficial.
OTHER PRIMARY CANCER SITES IN ADULTS
AND OUTCOME FOR CHILDHOOD CANCERS
In addition to breast and prostate cancer studies, data from prospective randomized trials and retrospective reviews of a number of other cancer sites fail to support the independent prognostic significance of race.57–61
A large study of childhood cancers reported by investigators from the St. Jude Children's Research Hospital demonstrated rather conclusively that with “equal access to effective contemporary” care, Black children have the same outcomes as White children. This study included more than 5,000 Black and White children treated for cancer between January 1962 and June 1992. These investigators noted that in the early years, Blacks had a lower survival rate largely due to more advanced disease at the time of diagnosis, but in more recent years there was no difference in outcome by race.61 Thus, as with adults, the preponderance of evidence suggests that race is not an independent prognostic factor for survival from childhood cancer.
IMPLICATIONS OF EDB
Following an extensive review of the available published data, race does not appear to be a major independent prognostic factor for survival from cancer. Instead, an epidemiologic phenomenon, EDB, may explain all or much of the apparent discrepancy in survival. This phenomenon is due to two related factors. First, the available staging systems are crude measures of the true extent or severity of disease. These staging systems are designed primarily to predict whether patients are curable and not specifically to predict the duration of survival. This limitation is most obvious when applied to patients with advanced cancer. Second, differences in the extent of disease distribution in different populations create bias when the groups are compared. This bias persists despite crudely correcting for stage. The EDB tends to create the general impression that race is an independent prognostic factor for survival from cancer. Careful analysis of the available epidemiologic studies
The assumption that race is an independent factor (without an adequate scientific basis) technically can be considered as “racist,” just as assuming that Black children score lower on standardized tests because of race is racist. The assumption that race is an independent prognostic factor deprives the “victims” of the opportunity to rectify the real health care problems. If the major problem is actually quality of care, efforts should be directed there. Differences in socioeconomic status and the variations in practice patterns and health outcomes by region of the country create enough doubt about the importance of race to require that any study that proposes to demonstrate differences in outcome by race be reviewed very critically for several reasons.7,20,22–24 First, the bulk of the literature suggests that such a study is likely to be flawed even if the source of the flaw is unclear. Second, thus far, the designation of race as a major health care variable has not resulted in the improvement of care for anyone. Those who have benefited the most from such practices have been health care researchers funded to do research on racial differences. Finally, nothing can be done about an individual's race.
If socioeconomic factors (lack of education, unemployment, and poor-quality health care) are the most important factors, money currently spent studying cancer outcome as a function of race might be better spent addressing these issues.62–64 Studies may also be considered to address the possibility of higher levels of environmental carcinogens in Black communities. Such studies would force us to study the impact of racism on health care delivery as a social disease. The political implications arising from these issues may not be as popular as acknowledging that “Blacks do worse,” as has been done for more than 20 years. Awareness of EDB may allow a more accurate assessment to be made of the impact of lack of education, environmental exposures, socioeconomic status, and other factors, such as diet, that may be associated with differences in survival from cancer.64
RACE AND CARDIOVASCULAR
DISEASE AND OTHER CAUSES OF DEATH
It is clear from the previous discussion that the preponderance of evidence suggests that race is not likely to be an independent prognostic
| Author (Reference Number) | Journal | Topic | Conclusions |
|---|---|---|---|
| Fang et al., 1996 (65) | New England Journal of Medicine | Study of the relationship between the place of birth and the cardiovascular mortality among non-Hispanic Black and White residents of New York City | Although Blacks born in the South had substantially higher age-adjusted rates of death from cardiovascular causes, both Caribbean- and northeastern-born Blacks ≥65 years had lower rates. |
| Krieger and Sidney, 1996 (66) | American Journal of Public Health | Association between blood pressure and self-reported experiences of racial discrimination and responses to unfair treatment | Black-White differences in blood pressure were reduced by accounting for reported experiences of racial discrimination and responses to unfair treatment. |
| Ayanian et al., 1993 (67) | Journal of the American Medical Association | Likelihood of coronary revascularization procedures among Medicare Part A enrollees | Whites more likely to receive such procedures. |
| Peterson et al., 1994 (68) | Journal of the American Medical Association | Assessment of cardiac catheterization or revascularization procedures among Blacks and Whites admitted with an acute myocardial infarction to Veterans Administration hospitals | Blacks 33%, 42%, and 54% less likely to undergo cardiac catheterization, undergo coronary angioplasty, and receive coronary bypass surgery, respectively, than Whites. |
| Kahn et al., 1994 (69) | Journal of the American Medical Association | Comparison of hospital care among elderly Black or poor Medicare recipients using a representative sample from 9,932 patients | Patients who are Black or poor have worse processes of care and greater instability at discharge. |
― 283 ―
Geronimus et al.,1996 (70) | New England Journal of Medicine | Study of the excess mortality among Blacks and Whites under the age of 65. | The probability of reaching age 65 was only 62% for Blacks compared to 77% for Whites, but there were large variations nationally such that Blacks from the Queens-Bronx had a higher likelihood of reaching age 65 than Whites from the lower east side, Detroit, the Appalachians, and northeastern Alabama. |
What about cardiovascular diseases, the number one cause of death? Fang et al. reported that the risk of death from cardiovascular disease appears to depend more on place of birth than on race.65 But how does one explain the differences seen in the incidence and severity of disease? The study by Krieger et al. may shed light on this matter.66 They reported that experiencing racial discrimination was associated with having a higher blood pressure. Several studies suggest that Whites tend to be treated more aggressively than Blacks with the same severity of disease.67 Recent studies have also shown that race was shown to influence the quality of hospital care received for cardiovascular illness regardless of whether patients were treated covered by Medicare or were treated within the Veterans Administration system.68,69 Still other studies demonstrate
CONCLUSIONS ABOUT RACE AND SCIENCE
Herein data have been presented that support the notion that an epidemiologic phenomenon, EDB, is likely to explain the lower survival rates noted for some “races” with cancer of several common sites. Race also does not appear to be an independent prognostic factor for survival from cardiovascular disease or overall mortality when adjustments are made for other confounding variables. It should not be assumed that biologic differences exist between people of different races unless there are very strong data to support this assumption. This conclusion provides a basis for designing strategies for addressing the problem of excess mortality seen in Blacks.
In addition to the fact that the overview of available medical data really does not support the notion that race is an independent prognostic factor, there is another major reason to doubt the notion that race has major independent significance. “Race” is not a true scientific biologic construct but rather a political construct for the purposes of conveniently dividing people. Recent review articles published in mainstream journals leave little to no doubt of this fact.73–77 For example, Paul Hoffman, in an editorial written for Discover magazine, summarized his findings of a series of review articles on race by several authorities: 73–76
On average there is a 0.2 percentage difference in the genetic material between any two randomly chosen people on Earth. Of diversity, 85 percent will be found within any local group of people—say, between you and your neighbor. More than half (9 percent) of the remaining 15 percent will be represented by differences between ethnic and linguistic groups within a given race (for example, between Italians and French). Only 6 percent represents differences between races (for example, between Europeans and Asians). And remember—that's 6 percent of 0.2 percent. In other words, race accounts for a minuscule 0.012 percent difference in our genetic material.
With such a small portion of our genetic makeup, why should anyone expect race to determine one's risk of dying of breast cancer, prostate cancer, hypertension, gunshot wounds, having a lower I.Q., and
genetic variation from one individual to another of the same “race” swamps the average differences between racial groupings. The more we learn about humankind's genetic differences, says geneticist Luca Cavalli-Sforza of Stanford University, who chairs the committee that directs the biodiversity project, the more we see that they have almost nothing to do with what we call race.77
Even today, research directed at continuing to prove the superiority of the “White race” continues to be funded.78 Although in this “free country” it may be reasonable to continue to allow privately funded racist research, it is inappropriate to continue to use taxpayer dollars to do so. Such research only serves to divide us as a nation. The mentality behind this type of race-based research was used to justify slavery some 400 years ago and the Tuskegee experiments more than 50 years ago.
BLAME, DO NOT COMPENSATE AND BAN:
RACE HEALTH AND PROPOSITION 209
The response of our society to its past moral and ethical failures to address the health needs of some of its people has been to first deny any responsibility for it. Blame the victims of race, and then do not compensate them—not only that, outlaw compensation and accuse them of “reverse discrimination.” Carl T. Rowan brought attention to this issue in his book The Coming Race War in America: A Wake-Up Call, when he wrote,
White male paranoia has become epidemic. This despite the fact that the median net worth of black households in this country is $4,604, or just one tenth the median net worth of white families—$44,408. The comparable figure for Hispanics is $5,345.79
The passage of Proposition 209 in California is but one example of how well-meaning people can be mislead into supporting an unjust cause that
On talk shows and elsewhere I am frequently asked why “blacks get all the college scholarships.” The General Accounting Office reports that 96 percent of all the scholarship money in America goes to whites has done little to wipe out white cries of persecution.79
The passage of Proposition 209 in California is likely to do more to continue to widen the gap between “the haves and the have-nots” and consequently to do more to widen the gap between the state of health for Blacks and Whites in California. The matter of Proposition 209 brings to mind the letter written to the clergymen on April 16, 1963, from a Birmingham jail by Martin Luther King Jr.:
There are some instances when a law is just on its face and unjust in its application. For instance, I was arrested Friday on a charge of parading without a permit. Now there is nothing wrong with an ordinance which requires a permit for a parade, but when the ordinance is used to preserve segregation … it becomes unjust.80
Much of what is currently believed about race in this country grows out of the same mentality that supported segregation then and race-based research now. This mentality is largely responsible for the state of health of African Americans today. In what is considered his last, and most radical, Southern Christian Leadership Conference (SCLC) presidential address, Martin Luther King Jr. raised the question in the title of his presentation: “Where Do We Go from Here?” 81
FUTURE DIRECTIONS:
“WHERE DO WE GO FROM HERE?”
I have argued that “race” is not inexplicably related to health but that racism and poverty are. The history of racial oppression of African Americans in this country is old, deep, and solidly entrenched in our literature, our science, and our culture.82 Racism and poverty have locked the vast majority of this population not in chains but in a complex superstructure whose roots reach back into the 1500s but whose branches still blossom and provide fruit of despair. This fruit of despair brings to mind the saying from the Tao Te Ching of Lao Tzu:
| The best lock has no bolt, and no one can open it. | |
| The best knot uses no rope, and no one can untie it.83 |
What can be more effective than “scientific proof” of racial differences as an explanation for being less healthy, less intelligent, and less hard working, even if this “scientific proof” is not valid?
When attempting to answer the question of where we go from here, Martin Luther King Jr. had this to say:
One night, a juror came to Jesus and he wanted to know what he could do to be saved. Jesus didn't get bogged down in the kind of isolated approach of what he shouldn't do. Jesus didn't say, “Now Nicodemus, you must stop lying.” He didn't say, “Nicodemus, you must stop cheating if you are doing that.” He didn't say, “Nicodemus, you must not commit adultery.” He didn't say, “Nicodemus, now you must stop drinking liquor if you are doing that excessively.” He said something altogether different, because Jesus realized something basic—that if a man will lie, he will steal. And if a man will steal, he will kill. So instead of just getting bogged down in one thing, Jesus looked at him and said, “Nicodemus, you must be born again.”
Here I would contend that similarly America needs to be “born again” with regard to its attitude toward race. We must end racist conjecture. Dr. King went on reflecting on the words of Jesus, saying,
He said, in other words, “Your whole structure must be changed.” A nation that will keep people in slavery for 244 years will “thingify” them—make them things. Therefore they will exploit them, and poor people generally, economically. And a nation that will exploit economically will have to have foreign investments and everything else, and will have to use its military might to protect them. All of these problems are tied together. What I am saying today is that we must go from this convention and say, “America, you must be born again!”
… let us go out with a “divine dissatisfaction.” Let us be dissatisfied until America will no longer have a high blood pressure of creeds and an anemia of city of wealth and comfort. … Let us be dissatisfied until those that live on the outskirts of hope are brought into the metropolis of daily security … and every family is living in a decent sanitary home.81
So, too, let us be dissatisfied as long as the risk of dying of cancer is 50% greater for Blacks compared to Whites. It is not due to race. We must end racism by first acknowledging this fact. Only then can we break the link between “race” and “health.”
NOTE
The author would like to acknowledge Marion Malack and Pamalar Lewis for editorial support and Dr. Deborah Roach for her patience. A special thanks to
REFERENCES
1. The Random House College Dictionary. Revised ed. 1975. New York: Random House.
2. Roach, M., Alexander, M., and Coleman, J.1992. “The prognostic significance of race on survival from laryngeal carcinoma.” Journal of the National Medical Association84, 668–674.
3. Jones, James H.1981. Bad Blood: The Tuskegee Syphilis Experiment.New York: The Free Press, 34.
4. Nortzon, F. C., Komarov, Y. M., Ermakov, S. P., et al. 1998. “Causes of declining life expectancy in Russia.” Journal of the American Medical Association279, 793–800.
5. “National Cancer Institute.” 1993. SEER cancer statistics review: 1973–1990. In NIH Publication 93-2789. Bethesda, Md.: National Cancer Institute.
6. Jones, James H.1981. Bad Blood: The Tuskegee Syphilis Experiment, New York: The Free Press, 38.
7. Moul, J. W., Douglas, T. H., McCarthy, W. F., and McLeod, D. G.1996. “Black race is an adverse prognostic factor for prostate cancer recurrence following radical prostatectomy in an equal access health care setting.” Journal of Urology155, 1667–1673.
8. Bauer, J. J., Connelly, R. R., Sesterhenn, I. A., et al. 1997. “Biostatistical modeling using traditional variables and genetic biomarkers for predicting the risk of prostate carcinoma recurrence after radical prostatectomy.” Cancer79, 952–962.
9. Gould, Stephen J.1996. The Mismeasure of Man. 2nd ed. New York: W. W. Norton.
10. Franklin, J. H., and Moss, A. A.1994. From Slavery to Freedom: A History of African Americans. 7th ed. New York: McGraw-Hill, 41.
11. Curtin, P. D.1969. The Atlantic Slave Trade: A Census.Pp. 275–276. Madison: University of Wisconsin Press.
12. Harley, S.1995. “The timetables of African-American history.” In S. Harley, ed., A Chronology of the Most Important People and Events in African-American History.Pp. 226–275. New York: Simon and Schuster.
13. Satariano, W. A., Belle, S. H., and Swanson, G. M.1986. “The severity of breast cancer at diagnosis: A comparison of age and extent of disease in black and white women.” American Journal of Public Health76, 779–782.
14. Bassett, M. T., and Krieger, N. K.1986. “Social class and black-white differences in breast cancer survival.” American Journal of Public Health76, 1400–1403.
15. Austin, J.-P., Covery, K., and Rotman, M.1990. “Age-race interaction in prostate adenocarcinoma treated with external irradiation.” International Journal of Radiation Oncology Biology Physics19 (Suppl. 1), 200.
16. Dayal, H., Power, R., and Chui, C.1982. “Race and socio-economic status in survival from breast cancer.” Journal of Chronic Diseases27, 675–683.
17. Ernster, V. L., Selvin, S., Sacks, S. T., et al. 1978. “Prostatic cancer: Mortality and incidence rates by race and social class.” American Journal of Epidemiology107, 311–320.
18. Keirn, W., and Meter, G.1985. “Survival of cancer patients by economic status in a free care setting.” Cancer55, 1552–1555.
19. Page, W. F., and Kuntz, A. J.1980. “Racial and socioeconomic factors in cancer survival.” Cancer45, 1029–1040.
20. Ruffer, J. E., Barry, E. E., Terry, P., et al. 1991. “Lower radiation dose may account for decreased survival of blacks with prostate cancer: Results of the 1978 patterns of care study.” International Journal of Radiation Oncology Biology Physics21 (1), 212.
21. Diehr, P., Yergan, J., Chu, J., et al. 1989. “Treatment modality and quality differences for black and white breast cancer patients treated in community hospitals.” Medical Care27, 942–958.
22. Egbert, L. D., and Rothman, I. L.1977. “Relationship between race and economic status of patients and who performs their surgery.” New England Journal of Medicine297, 90–91.
23. Flaherty, J. A., and Meagher, R.1980. “Measuring racial bias in inpatient treatment.” American Journal of Psychiatry137, 679–682.
24. McWhorter, W. P., and Mayer, W. J.1987. “Black-white differences in type of initial breast cancer treatment and implications for survival.” American Journal of Public Health77, 1515–1517.
25. National Institutes of Health.1994. National Institutes of Health Guide for Grants and Contracts23 (11, March 18), 2.
26. Roach, M., and Alexander, M.1995. “The prognostic significance of race and survival from breast cancer.” Journal of the National Medical Association87, 214–219.
27. 1997. AJCC Cancer Staging Manual. 5th ed. New York: Lippincott-Raven.
28. Rosen, P. P., Groshen, S., Saigo, P. E., et al. 1989. “A long term follow-up study of survival in stage I (T1N0M0) and stage II (T1N1M0) breast carcinoma.” International Journal of Radiation Oncology Biology Physics7, 355–366.
29. Albain, K. S., Crowley, J. J., LeBlanc, M., et al. 1991. “Survival determinants in extensive-stage non-small-cell lung cancer: The Southwest Oncology Group experience.” Journal of Clinical Oncology9, 1618–1626.
30. Mountain, C. F.1986. “A new international staging system for lung cancer.” Chest89, 225S-233S.
31. Mittra, N. K., Rush, B. F., and Verner, E.1980. “A comparative study of breast cancer in the black and white populations of two inner-city hospitals.” Journal of Surgical Oncology15, 11–17.
32. Gregorio, D. I., Cummings, K. M., and Michalek, A.1983. “Delay, stage of disease, and survival among white and black women with breast cancer.” American Journal of Public Health73, 590–593.
33. Valanis, B., Wirman, J., and Hertzberg, V. S.1987. “Social and biological factors in relation to survival among black vs.” white women with breast cancer. Breast Cancer Research and Treatment9, 134–144.
34. Polednak, A. P.1988. “A comparison of survival of black and white female breast cancer cases in upstate New York.” Cancer Detection and Prevention11, 245–249.
35. Dansy, R. D., Hessel, P. A., Browde, S., et al. 1988. “Lack of a significant independent effect of race on survival in breast cancer.” Cancer61, 1908–1912.
36. Sutherland, C. M., and Mather, F. J.1988. “Charity hospital experience with long-term survival and prognostic factors in patients with breast cancer with localized or regional disease.” Annals of Surgery207, 569–580.
37. Fields, J. N., Kuske, R. R., Perez, C. A., et al. 1989. “Prognostic factors in inflammatory breast cancer.” Cancer63, 1232–1255.
38. Cella, D. F., Orav, E. J., Kornblith, A. B., et al. 1991. “Socioeconomic status and cancer survival.” Journal of Clinical Oncology9, 1500–1509.
39. Axtell, L. M., and Myers, M. H.1978. “Contrasts in survival of black and white cancer patients, 1960–1973.” Journal of the National Cancer Institute60, 1209–1215.
40. Farrow, D. C., and Hunt, S. J. M.1992. “Geographic variation in the treatment of localized breast cancer.” New England Journal of Medicine326, 1097–1101.
41. Albain, K. S., Green, S., LeBlanc, M., et al. 1992. “Proportional hazards and recursive partitioning and amalgamation analyses of Southwest Oncology node-positive adjuvant CMFVP breast cancer data base: A pilot study.” Breast Cancer Research and Treatment22, 273–284.
42. Roach, M., III, Cirrincione, C., Budman, D., et al. 1997. “Race and Survival from Breast Cancer: Based on Cancer and Leukemia Group B Trial 8541.” The Cancer Journal of Scientific American3, 107–112.
43. Dignam, J. J., Redmond, C., Fisher, B., et al. 1997. “Prognosis among African-American women and white women with lymph node negative breast carcinoma: Findings from two randomized and clinical trials of the National Surgical Adjuvant Breast and Bowel Project (NSABP).” Cancer80 (1), 80–90.
44. Krieger, N.1989. “Exposure, susceptibility, and breast cancer risk.” Breast Cancer and Treatment13, 205–223.
45. Morabia, A., and Wynder, E. L.1990. “Epidemiology and natural history of breast cancer.” Surgical Clinics of North America70, 739–752.
46. Eley, J. W., Hill, H. A., Chen, V. W., et al. 1994. “Racial differences in survival from breast cancer: Results of the National Cancer Institute Black/White Cancer Survival Study.” Journal of the American Medical Association272, 947–954.
47. Nattinger, A. B., Gottlieb, M. S., Verum, B. S., et al. 1992. “Geographic variation in the use of breast-conserving treatment for breast cancer.” New England Journal of Medicine326, 1102–1107.
48. Ragaz, J., Jackson, S. M., Plenderleith, I. H., et al. 1993. “Can adjuvant radiotherapy (XRT) improve the overall survival (OS) of breast cancer (BR CA) patients in the presence of adjuvant chemotherapy (CT)?” 10-year analysis of the British Columbia randomized trial. Proceedings of ASCO12, 70.
49. Natarajan, N., Murphy, G. P., and Mettlin, C.1989. “Prostate cancer in blacks: An update from the American College of Surgeons' patterns of care studies.” Journal of Surgical Oncology40, 232–236.
50. Perez, C. A., Garcia, D., Simpson, J. R., et al. 1989. “Factors influencing outcome of definitive radiotherapy for localized carcinoma of the prostate.” Radiotherapy and Oncology16, 1–21.
51. Roach, M., III, Krall, J., Keller, J. W., et al. 1992. “The prognostic significance of race and survival from prostate cancer based on patients irradiated on Radiation Therapy Oncology Group protocols (1976–1985).” International Journal of Radiation Oncology Biology Physics24, 441–449.
52. Epstein, J. I., Paull, G., Eggleston, J. C., et al. 1986. “Prognosis of untreated stage A1 prostatic carcinoma: A study of 94 cases with extended followup.” Journal of Urology136, 837–839.
53. Levine, R. L., and Wilchinsky, M.1979. “Adenocarcinoma of the prostate: A comparison of the disease in blacks versus whites.” Journal of Urology121, 761–762.
54. Roach, M., III. “Is race an independent prognostic factor for survival from prostate cancer?” Journal of the National Medical Association90 (11, suppl.).
55. Vijayakumar, S., Winter, K., Sause, W., et al. 1998. “Prostate specific antigen levels are higher in African-American patients than whites in a national registration study: Results of RTOG 94–12.” International Journal of Radiation Oncology Biology Physics40, 17–25.
56. Chamberlain, R., Winter, K., Vijayakumar, S., et al. 1998. “Radiation Therapy Oncology Group demographic analysis of subjects in multicenter clinical trials.” International Journal of Radiation Oncology Biology Physics40, 9–15.
57. Graham, M. V., Geitz, L. M., Byhardt, R., et al. 1992. “Comparison of prognostic factors and survival among black and white patients treated with radiation therapy for non-small cell lung cancer.” Journal of the National Cancer Institute84, 731–735.
58. Simpson, J. R., Scott, C. B., Curran, W. J., et al. 1993. “Race, gender and socioeconomic status of brain tumor patients entering multicenter clinical trials—A report from the Radiation Oncology Group.” International Journal of Radiation Oncology Biology Physics26, 239–244.
59. Streeter, O. E., Martz, K. L., Gaspar, L. E., et al. 1999. “Does race influence survival for esophageal cancer?” An analysis of patients treated with chemoradiation on RTOG 85–01. International Journal of Radiation Oncology Biology Physics44 (5), 1047–1052.
60. Modiano, M. R., Villar-Werstler, P., Crowley, J., et al. 1996. “Evaluation of race as a prognostic factor in multiple myeloma: An ancillary of Southwest Oncology Group Study 8229.” Journal of Clinical Oncology14, 974–977.
61. Pui, C.-H., Boyett, J. M., Hancock, M. L., et al. 1995. “Outcome of treatment for childhood cancer in black as compared with white children: The St. Jude Children's Research Hospital experience, 1962 through 1992.” Journal of the American Medical Association273, 633–637.
62. Gabel, L. L., and Weddington, W. H.1993. “Obligation and opportunity: Family practice research regarding race and quality of care.” Family Practice Research Journal13 (2), 101–104.
63. Weddington, W. H., Gabel, L. L., Peet, G. M., et al. 1992. “Quality of care and black American patients.” Journal of the National Medical Association84 (7), 569–575.
64. Gorey, K. M., and Vena, J. E.1994. “Cancer differentials among U.S. blacks and whites: Quantitative estimates of socioeconomic-related risks.” Journal of the National Medical Association86 (3), 209–215.
65. Fang, J., Madhavan, S., and Alderman, M.1996. “The association between birthplace and mortality from cardiovascular causes among black and white residents of New York.” New England Journal of Medicine335 (21), 1545–1551.
66. Krieger, N., and Sidney, S.1996. “Racial discrimination and blood pressure: The CARDIA Study of young black and white adults.” American Journal of Public Health86, 1370–1378.
67. Ayanian, J. Z., Udvarhelyi, S., Gatsonis, C. A., et al. 1993. “Racial differences in the use of revascularization procedures after coronary angiography.” Journal of the American Medical Association269 (20), 2642–2646.
68. Peterson, E., Wright, S. M., Daley, J., and Thibault, G. E.1994. “Racial variation in cardiac procedure use and survival following acute myocardial infarction in the Department of Veterans Affairs.” Journal of the American Medical Association271, 1175–1180.
69. Kahn, K. L., Pearson, M. L., Harrison, E. R., et al. 1994. “Health care for black and poor hospitalized medicare patients.” Journal of the American Medical Association271, 1169–1174.
70. Geronimus, A. T., Bound, J., Waidmann, T. A., et al. 1996. “Excess mortality among blacks and whites in the United States.” New England Journal of Medicine335, 1552–1558.
71. Hafner-Eaton, C.1993. “Physician utilization disparities between the uninsured and insured: Comparisons of the chronically ill, acutely ill and well non-elderly populations.” Journal of the American Medical Association269, 787–792.
72. Worthington, C.1992. “An examination of factors influencing the diagnosis and treatment of black patients in the mental health system.” Archives of Psychiatric Nursing6 (3), 195–204.
73. Hoffman, P.1994. “The science of race.” Discover: The World of Science15 (4, Special Issue), 4.
74. Gould, S. J.1994. “The geometer of race.” Discover: The World of Science15 (4, Special Issue), 64–69.
75. Gutin, J. A.1994. “End of the rainbow.” Discover: The World of Science15 (4, Special Issue), 70–75.
76. Diamond, J.1994. “Race without color.” Discover: The World of Science15 (4, Special Issue), 82–89.
77. Begley, S.1995. “Three is not enough.” Newsweek, February 13, 67–69.
78. Miller, A.1994/1995. “The Pioneer Fund: Bankrolling the professors of hate.” Journal of Blacks in Higher Education, no. 6(winter), 58–61.
79. Rowan, C. T.1996. The Coming Race War in America.Boston: Little, Brown, 17.
80. King, Martin Luther, Jr.1991. “Historic essays: Letter from Birmingham City Jail (written April 16, 1963).” In A Testament of Hope: The Essential Writings and Speeches of Martin Luther King, Jr., edited by James M. Washington. San Francisco: HarperCollins, 294.
81. King, Martin Luther, Jr.1991. “Famous sermons and public addresses: Where Do We Go From Here? (written April 1967).” In A Testament of Hope: The Essential Writings and Speeches of Martin Luther King, Jr., edited by James M. Washington. San Francisco: HarperCollins, 251.
82. Morrison, T.1993. Playing in the Dark: Whiteness and the Literary Imagination.New York: Vintage Books.
83. Brown, B., trans.1995. The Tao Te Ching of Lao Tzu.New York: St. Martin's Press, Saying 27.