9. Social Organization
Social organization can be broadly defined as the pattern of interactions between individuals. This pattern determines ecological questions, such as how the physical space of the habitat is used by different classes of individuals and how resources are allocated among them; and also more behavioral and evolutionary questions, such as which individuals produce offspring and what happens to those offspring as they mature. Because treeshrews could not often be watched, I had to infer their social relationships from the deployment of their home ranges and the relative movements of radio-tagged and trapped individuals. The key question of whether treeshrew home ranges also constituted territories had to be judged indirectly. As I could not see territorial defense behaviors, I define territoriality simply as the exclusive use of a space by a class of individuals, to the exclusion of others of the same class. If a space were not defended in some fashion, I assume that other individuals would use it.
SOCIAL RELATIONS OF TREESHREW SPECIES
PTILOCERCUS LOWIILim (1967) reported that pentails nest in malefemale pairs, and Muul and Lim (1971) found up to five pentails in the same nest, but they did not report their sexes or ages. Four pentail specimens collected by Muul and Lim on the same day in the same locality, preserved in the National Museum of Natural History (USA), include an old adult male and an old adult female of about 50 g each and two
The first two pentails captured at Danum Valley were nonparous females with unworn, but complete, adult dentition (F96, 43 g, Nov. 1990; F163, 53 g, Mar. 1991), and within the weight range cited for adults (40–60 g; Gould 1978). These were followed by captures of a young adult male (50 g) with slightly worn teeth (July 1991) and, finally, of an adult, larger, lactating female with well-worn teeth (F181, 63 g, Aug. 1991). Each was captured after the radio of the previous one had quit (although transmitters exceeded specifications and ran for 90 to 100 days). The radio on the male slipped off in the middle of the first night of tracking, so I logged only a few data points. This species was difficult to trap, and only one animal was recaptured (once).
These four pentails denned by day in the same hollow tree (see chap.6) and were members of the same group. The old female F181 was likely the mother of the two subadult females, but the male could have been either her son or her mate. The nest tree had multiple exits, and I counted up to four “large” pentails emerging from the tree at nightfall, but more often I saw only one to three. Pentails whisked at high speed around and around and up and down trunks and vines, materializing and vanishing unpredictably, so it was impossible to be sure of a count unless all were audible/visible at once. Radio-tagged pentails often left the tree out of sight, from hidden canopy holes, and other individuals must have done likewise. Thus it was impossible to ascertain how many were living together, but there was always more than one in the tree.
When they left the nest tree at nightfall or returned to it at midnight or dawn, the pentails often briefly ran about together, one following another around the trunk of the nest tree and through the adjacent midand understory vegetation. At these times they were likely to chirp with birdlike trills. Some calls were evidently in alarm, directed at me, and these were most often elicited near the nest tree when a number of pentails were about. Several often emerged together, and they returned from foraging quite synchronously. In contrast, of more than forty observations of pentails away from the nest tree, all were of solitary animals, and all individuals radio-tracked always foraged alone. Pentails therefore socialized at home, in or beside the colony tree, but foraged solitarily.
The members of the group of pentails had a remarkable arrangement of home ranges. The two young females had home ranges mostly within the larger home range of their presumed mother, as is usual among mammals, but the two youngsters used ranges almost entirely exclusive of each other, like opposite spokes of a wheel from the nest hub. The male, in his half-night record, went straight to an unused sector between the ranges of the young females but also within the area of the old female (fig. 9.1). The parous female in turn spent most of her time in areas far from the nest, outside those used by the young, although one small zone was heavily used by young F163 and the mother (fig. 9.1). Because I cannot be certain that the previously radio-tagged pentail was still one of those in the colony when the next one was followed (although I believe that they usually were), this intriguing pattern needs further confirmation.
While tracking a pentail, I occasionally saw another, but there was no way of knowing if these were from the same or different groups. I saw no chases. The apparent repulsion between ranges of the young hints of exclusive foraging domains for each individual, within a group domain outlined by the home range of the adult female, but I have no data on whether the colony itself, or the adult female, was territorial. When Gould (1978) mixed strange adult pentails together in a room, a male and female did not fight, while two females at first were aggressive and fought with biting but eventually nested together.
TUPAIA MINOR Although T. minor was the most commonly seen species, we captured relatively few: seven at Poring, of which four were adults, and eleven at Danum, of which ten were adults. At Poring we radio-tagged one adult female (F112). A young adult male with unworn teeth that was radio-collared in her home range (M126) promptly moved to a separate area, and he was probably a dispersing young. At Danum I radio-collared a pair (F70, M91) and, eventually, a neighboring female (F294). Several others were fitted with bead collars, and these, or juveniles with tail-hair clips, were spotted from time to time on both study sites.
The pair of T. minor at Danum Valley used almost perfectly coincident home ranges (fig. 9.2). That of the male was slightly smaller than that of the female, and entirely within it. The male was captured two months after the female, when her radio was failing, so their interactions were recorded only during his tracking sample. These two animals met often, usually three to four times a day, and on 7 December they were near each other most of the day while they traveled 980 m, with F70 recorded close to M91 thirteen times. They foraged together for an hour
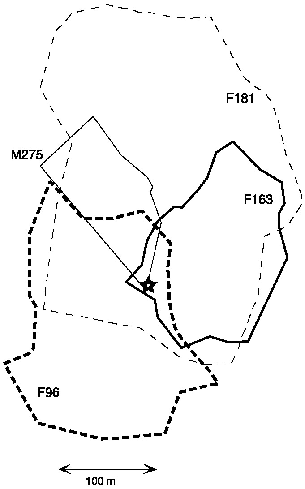
Fig. 9.1. Home ranges of the four pentail treeshrews. Star = the communal nest tree. M275 was followed for only half a night. Note separated ranges of F96 and F163, young females assumed to be littermates, although not followed simultaneously (see text). F181 was an adult, lactating female and the presumed mother of the other females.
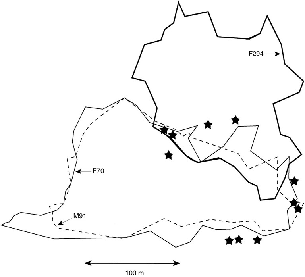
Fig. 9.2. Territories of a pair of T. minor (male M91 and female F70) and a female neighbor (F294, heavy line). Stars are locations where unmarked T. minor were seen during radio-tracking of members of the pair. At that time F294 had not been captured and she could have been among them.
Treeshrew groupings that I saw throughout the study, including while radio-tracking (table 9.1), show that lesser treeshrews were the most likely to be in pairs. Almost a third of individuals were noted in general proximity to another T. minor, but because they were not usually close together, associations were not always clear. The male M91 appeared to be monogamous, because he was restricted to the range of F70, but his female's range may have overlapped those of other males as it was slightly larger.
When a pair of T. minor rested in the same fruit tree, they were always far apart, just as they were when foraging. I saw a male sniff and mark and then use the favorite resting perch of F112 in a fruit tree, where she usually rested several hours each day. Afterward she seemed to stay away from this spot, although she did continue to rest there from time to time.
| Species | N | Solitary | Twos, Total(%) | Two at Fruit | Two Chase | Female + Young | Other Pairs |
|---|---|---|---|---|---|---|---|
| NOTE: Number of sightings of single Tupaia or two seen fairly close together in time and space. Some of these may not have been associated. N = number of treeshrews; numbers in other columns are the number of observations (for twos, there are twice as many animals as observations). Fruit = seen at a fruit tree; therefore association may be coincidental. Chases were agonistic. More than two were never seen together. Includes treeshrews seen while radio-tracking but not T. tana F109 with her young. | |||||||
| T. minor | 176 | 128 | 24 (27) | 7 | 1 | 16 | |
| T. gracilis | 74 | 64 | 5 (14) | 1 | 4 | ||
| T. longipes | 76 | 70 | 3 (8) | 1 | 2 | ||
| T. tana | 107 | 95 | 6 (11) | 1 | 5 | ||
The two neighboring females, F70 and F294, had almost exclusive ranges, and I consider them to have been territorial. They overlapped on the northeast section of F70's range, but it is noteworthy that the area of overlap was not used by F70's male, M91 (fig. 9.2) and that F70 only used this area slightly. Merely 14 of 207 recorded radio-tracking points were within it. These were recorded in October 1990, and she was not registered on the area in December when M91 was followed. F294 was not followed until much later, in June 1991 (when F70 was still present but without a radio), so it is not certain that there was any simultaneous home range overlap. The only aggressive interaction that I saw between lesser treeshrews (unidentified individuals) was exactly in the corner of that trail quadrant where the two female ranges appear to overlap, evidence that the sector was in dispute. The sites where I saw unmarked lesser treeshrews while radio-tracking M91 (stars in fig. 9.2) were all outside the periphery of his and his female, F70's, home ranges, which supports the hypothesis of territoriality.
TUPAIA GRACILIS Slender treeshrews were the rarest of the Tupaia captured; only three adults and four or five subadults (one escaped) were trapped on the East Ridge at Poring, and five adults and four subadults were trapped at Danum. At Poring, the home range of the single female collared (F105) was 660 m long and 191 m wide and covered the whole study area. No other adult female was captured, so her range seemed to be exclusive. One male was captured and radio-tagged on her range and was recorded meeting with her, but some mishap occurred, because before he could be followed, his signal became fixed at the bottom of a ravine off the study area, implying that he had died. When the area was
At Danum, only a single adult, female T. gracilis F67, was trapped on the study plot in the first six months from September to March, but I sighted at least one other animal, which could have been the one subadult that was also captured. In late March another female (F173) and two males appeared (M175, Mfoot). All were radio-collared, but the signal of M175 was never received, he was not recaptured, and he may have been a transient. Mfoot was badly injured in the trap and snapped his hind leg completely across above the heel. I immediately released him as he was, without marking or handling, and was amazed and delighted when we recaptured him several times, gradually healing, during the next months. His leg had healed by June, but the foot had lost most toes and was deformed and nearly useless. I radio-tagged him in June and followed him through August. This astonishing three-legged male, of only 80 g, had the largest home range recorded in a treeshrew and nearly the largest daily movements (see table 8.1).
Slender treeshrews appeared to be territorial, with only one adult of each sex present on a home range. The male M175, whose radio signal was never found, was captured on the north border of Mfoot's home range. Mfoot was last seen on 23 August, and on 24 September, the last day of trapping, another very young adult male was caught about 100 m inside his home range on the south side. The territory of Mfoot included the territories of both females F67 and F173 (fig. 9.3) and also extended to the west beyond these, and thus it could have included part of the range of another (but the trapline passed through that area, and none was caught there). The female F67 occupied the southern half of the study area from September 1990 to 19 March 1991. F173, on a day in April, used the contiguous northern half of the study area. Gradually, through April to June, F173 moved south. F67 stayed farther and farther south, until in her last records in mid-April, when her second radio quit, she was altogether south of the study area. F173 clearly displaced F67 in a gradual process lasting about a month. In her last tracking record, on 11 July 1991, F173 used much the same area used by F67 on 24 September 1990, so replacement seemed total. I do not know if F67 was then still alive.
The male T. gracilis and F173 both had functional radios when he was followed (but F67 did not). On every day he spent time in the territories of both females, looping from one to the other. On one of the five days he met F173, and his signal followed hers for 40 min as she moved; on
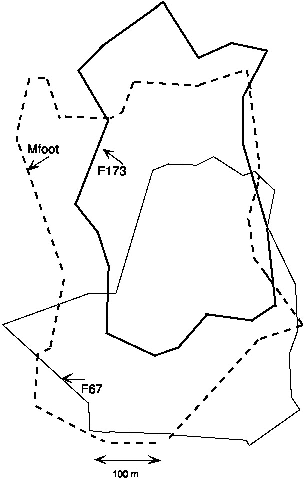
Fig. 9.3. Complete ranges of the three T. gracilis followed at Danum Valley. The two females, F67 and F173, did not spatially overlap in time, but F173 took over the zone of apparent overlap, displacing F67 toward the south (bottom), while the male (Mfoot) stayed in place, overlapping both females.
Slender treeshrews foraged solitarily and were rarely seen together (table 9.1). Radio-tracking showed that the male met the female F173 on most days, and he also went to where he would have found F67, so
TUPAIA LONGIPES On the East Ridge at Poring, only four plain treeshrews were captured, one subadult and three adults, two females and a male. All adults were radio-collared. At Danum Valley I captured twenty-six individuals: four adult males, five adult females, and the rest young, some of which grew to adulthood on the study area.
Tupaia longipes appeared to be strictly territorial; there was exact meeting but no overlap between the ranges of neighboring adults of the same sex (figs. 9.4, 9.5). The males had territories with most borders almost superimposed on those of the females, but in two of the three pairs for which males and females were simultaneously radio-tracked, the males used some extra territory. At Poring M138 went on a single excursion into an area well outside of the range of his mate, F132. Possibly this “annex” was not defended territory. He did not overlap at all with the neighboring female on the other side (F133). Territories were so large that only one fit on the main study area at Poring, and only two fit at Danum. I never was able to capture the adults that must have occupied the northwest side at Danum, although I trapped their presumed young. The northeast animals used such a vast area that they went out of receiver range and I would “lose” them. Because it was so difficult to get good data with complete daily records, I followed them little.
For one month, I radio-collared a subadult/just adult male, M79, which was trapped in the middle of the study area (Oct.–Nov. 1990), to see how he interacted with the resident adults. His peculiar, L-shaped home range fringed that of the adults, with clear avoidance of their territory (fig. 9.4). He entered their territory seemingly to visit the baited trap sites. He was never recaptured after his radio was removed in November.
In January 1991 a juvenile female (F86) born on the study area in September, and first captured in October (115 g), settled on the west side of the territory of F56. Because she was captured as a juvenile 150 m north of F56's territory, she was probably not a daughter, but the possibility cannot be excluded. This female appropriated almost half of F56's old territory, and F56 then used only the east side (fig. 9.5). This pattern persisted until the end of the study. I once saw these two females meet, and one chased the other, but I could not see which. The male of that territory
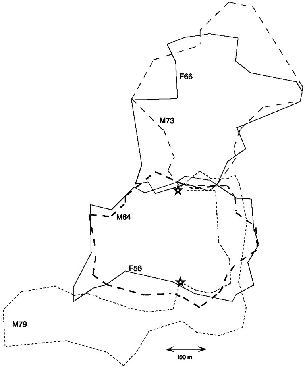
Fig. 9.4. Territories of two neighboring pairs of T. longipes at Danum Valley, September to December 1990, and the range of a subadult male, M79, during one month, October-November. The two stars indicate the location of trap sites that apparently drew M79 into the territory of M64.
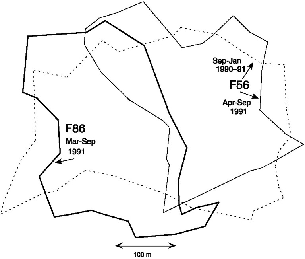
Fig. 9.5. Takeover in March 1991 of half of the territory of T. longipes female F56 by female F86, born on the study area in September 1990.
Males and females of Tupaia longipes pairs that shared territories met briefly from time to time. Long periods (hours) were sometimes spent together at fruit trees, perhaps coincidentally. The pair at Poring (F132, M138) had a behavior never detected in any other Tupaia individual of any species: they shared three of the same nest sites (burrows), but they used them alternately, never together. The members of the other two radio-tagged T. longipes pairs did not even have burrows in the same parts of their mutual home range.
Plain treeshrews were thus territorial within sexes, with males either monogamous or perhaps overlapping a second female. Female territories were so large that, as with slender treeshrews, it is unlikely that males
TUPAIA MONTANA Montane treeshrews were studied for only a month, so the data are a snapshot of a few individuals. We radio-tagged four females and two males and tracked for only two five-day samples, when we tried to follow several animals simultaneously.
The pattern of home ranges shows within-sex territories, with neighbor boundaries tightly following each other's contours (fig. 9.6). The single pair for which there were good data for both animals had almost perfectly congruent male and female borders, implying a monogamous pair. The incomplete data for two other pairs are consistent with the same configuration, but there is not enough information to know if the males all had one-female territories.
The T. montana pair (F50, M143) spent two of the ten days almost always together, an hour on another day, and a few minutes on most of the remaining days. Because this species had small territories, their extensive foraging travels folded back and forth in a compact space, and pairs were bound to meet often even if they traveled randomly. This male and female nested so close together on some nights that from a distance we thought that they were in the same nest, but when we worked our way in through difficult, steep terrain, we found them in separate nests 4 m apart. These limited results seem to show that mountain treeshrews resemble plain treeshrews in their land tenure system, but pairs seemed to spend more time close to each other.
TUPAIA TANA At Poring we trapped three adult female T. tana, one male and two subadults. We radio-tagged the male and two adult females. At Danum Valley we captured thirty-two individuals, of which about thirteen were subadults, and followed nine females and eight males by radiotelemetry. Because the array of animals was more complete, I describe the social organization only for Danum Valley, but the behavior of T. tana at Poring showed no salient differences.
As in every other Tupaia species followed, known resident adults of the same sex had nonoverlapping territories. Female territories in most
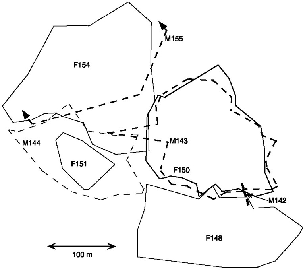
Fig. 9.6. Territories of all T. montana followed on the high plot at Poring. Note how the irregular range boundaries of females F148, F150, and F154 fit tightly together like pieces of a puzzle. Only one heterosexual pair is adequately represented (M143 and F150). Their ranges coincide almost perfectly.
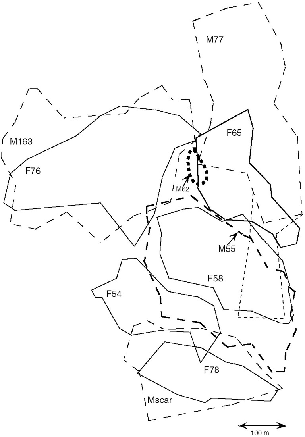
Fig. 9.7. Territories of nine adult T. tana at Danum Valley from September to December 1990. The location of one subadult male (M62, not radio-tagged) is also shown. Solid lines = females; dashed lines = males. The line of small dashes below the range of M77 represents a single excursion of one hour, which was probably outside of his territory.
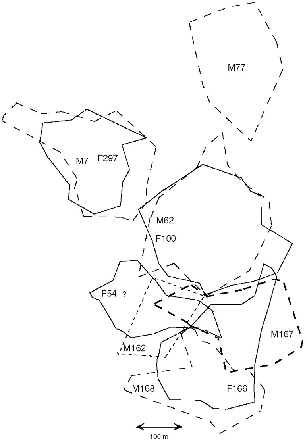
Fig. 9.8. The arrangement of territories of T. tana from March to September 1991. Solid lines = females; dashed lines = males. It is unknown whether female F54 was still present. Note the overlap of F166 with two males (M167 and M168). The intersection of several ranges on the east side of M162 is at some popular trap sites (see text). Because there were so many animals to follow, the data on individual treeshrews were collected in a time-staggered series, and because changes in territories happened at different times, not all the animals shown were present throughout all the months for which a figure is relevant. One female that had a recorded tenure of only a couple of months is not shown (although she had young and a territory), because it would give a false impression of overlap that did not exist.
Unlike other Tupaia, the territories of male and female large treeshrews coincided only approximately. Although most of the territory of a female was in six of eight cases in the home range of a single male, in every case the boundaries of males and females were not sharply concordant, and both males and females at least partially overlapped more than one member of the opposite sex. One male, M111 at Poring, probably overlapped two females, and M77 at Danum probably did. One female (F166) had most of the territories of two males in her territory (M167, M168; figs. 9.7, 9.8).
Both male and female large treeshrews thus had more than one possible overlapping mate, but most individuals were only recorded actually encountering the one animal of the opposite sex with whom they shared most of their territory. It was evident from following large treeshrews in the field that males were the active initiators of most encounters. As in the case of the lactating female F109 that we detailed at Poring (Emmons and Biun 1991), a male would travel to where a female was, usually early in the morning when she was foraging. He would spend from a few minutes to hours with her or trailing her (his signal following hers). The following pairs were registered as being together: F109 and M111 (on 7 days); F76 and M63 (2 days); F65 and M77 (5 days); F78 and Mscar (5 days); F297 and M7 (3 days). In addition, F100 was near M7 once, and F166 was near M162, but it was uncertain whether they met.
The most interesting behavior was that of F54 and Mscar, who were not principal overlapping mates (fig. 9.7). This male visited F54 on five days, and on three of these he also visited F78, sometimes more than once. As I followed him I was convinced that he would start looking for females, running without seeming to forage, first to where he would find F78 and then to the zones where he was likely to meet F54. These last places were on the extreme border of her home range, and she sometimes seemed to deliberately go and hang around there. F54 was once noted with another overlapping male, M55, under a fruit tree. Possibly these interactions with a neighbor male were due to the lack of her own mate. No known male overlapped most of her small territory, until her presumed son (M162) matured and stayed there. The way that territories fitted around the traplines precluded trapping T. tana on the southwest side, and it is possible but unlikely that F54 had a male that I did not catch. Because of the combined vicissitudes of trapping and timing
Large treeshrew pairs thus seemed to be more loosely associated than those of other species, and their territory deployment implied that they might be able to choose between several mates by choosing which parts of their territories to use.
Just as with T. gracilis and T. longipes, there was a changeover in T. tana individuals during the year, with a stable set of adults present from September to December/January and a new set that replaced some of these and formed a new distribution of home ranges, from March to September (figs. 9.7, 9.8). In this last half of the year, when many new residents became established, territories were smaller and the density of animals increased (fig. 9.8, table 8.5). Two subadult females and one subadult male, all probably born on the study area, became resident adults. The fate of most of the animals they replaced—whether they died or were forced off their territories—was unknown. Male M77 “lost” the southern part of his territory and was living only at its northern end in August 1991, and I presume an unmarked male that was seen in the vicinity had taken over the vacated zone.
territorial behaviors
During the entire study, I saw only four aggressive chases that could have been territorial fights. I saw treeshrews marking by rubbing parts of their bodies against the substrate only nine times: T. minor three times (two males, one female); T. tana four times (three males, one unknown sex); and slender and pentail treeshrews once each. Males dominated the tiny sample with six of seven records where sex was known or inferred.
DISCUSSION OF SOCIAL BEHAVIOR
social system
TUPAIA The only other description of the social organization of wild treeshrews is in the excellent and detailed study of T. glis on Singapore by Kawamichi and Kawamichi (1979, 1982). Their dense study population of about thirty resident adults of each sex had a social system virtually identical to those I found in the Tupaia species in Sabah. Of sixteen male territories they recorded, fourteen were monogamous, with one included female, and two were polygynous, one with two females and
Based on their behavior in captivity,Martin (1968) correctly inferred that Tupaia glis (= belangeri) lived in groups no larger than a family unit of two parents and their young. He surmised that the male and female were strongly pair-bonded, because captive pairs slept together in the same nest box, rested in contact, and marked each other. None of these behaviors was seen in the species studied in Sabah; but nonetheless, the concordance of territorial boundaries, and the frequent encounters between co-owners of territories, implies stable bonds between males and their one or more females, though not strict monogamy. Because the T. glis studied by Kawamichi and Kawamichi (1979) exhibited social organization apparently identical to that of T. longipes, T. minor, T. montana, and T. tana in Sabah, it is likely that the laboratory-bred stock of T. glis (of unknown origin) studied byMartin (1968) was not originally fundamentally different from other Tupaia and that the nest-sharing and other cohesive behaviors were artifacts of the close conditions of captivity.
The Kawamichis used the term “solitary ranging pair” for this social system of solitary animals that are monogamous on a common territory that each sex defends against its own gender. This has also been called Type I or “facultative” monogamy (Kleiman 1977). The Kawamichis cited a few other species with this system, including some nocturnal prosimians (Bearder 1987) and pikas (Ochotona spp.; Smith and Ivins 1984), but it is actually found in many mammals, including elephant shrews (Rathbun 1979), maned wolves (Dietz 1984), agoutis (Dubost 1988), and others (Kleiman 1977). There is no fundamental difference between solitary territories where a male overlaps one female and those where he may overlap one, two, or more, as in Tupaia species, except that as the system becomes more skewed (more females sequestered by each male), there are other developments such as sexual dimorphism and skewed reproductive success. This last may be one of the most common social organizations among “solitary” territorial mammals, such as galagos (Charles-Dominique 1977), ocelots (Emmons 1988), spiny rats (Emmons 1982), and probably hundreds of rodents. Little phylogenetic
Obligate, as opposed to facultative, monogamy in vertebrates such as birds (where it is the dominant system) and mammals such as foxes and small primates is generally agreed to be the result of increased offspring survivorship if both parents contribute to their provisioning or care (Kleiman 1977). I believe that in treeshrews and other species with facultative monogamy, where the father has no direct role in caring for the young, the defense of a territory where young remain until they reach adult size is a large indirect contribution to their nutrition. This may be as important to their survival as more direct provisioning in obligate pairs. If a male is able to defend two females, then each of their territories has only half a male feeding on it, which can only be to their advantage.
Arboreal, terrestrial, lowland, and montane species of Tupaia all had versions of the same social organization, but especially in T. gracilis and T. tana variations in the number of female territories on a male territory, or vice versa, and the deviations from exact concordance of male-female boundaries give both sexes possible access to several mates within the system of monogamous pair territories. It would be interesting to test the paternity of Tupaia young, to see how monogamous the social system really is.
PTILOCERCUS From all meager evidence, like Tupaia, pentail treeshrews seem to be monogamous but with a completely different social organization. Unlike Tupaia, pentails have a den-centered existence. An adult pair and their offspring always used a single nest site together and radiated their foraging centrifugally from it. If the pair lives together permanently with their young, they would seem to have cohesive, obligate or Type II monogamy. It is yet unproven that the male stays permanently with the female; and the length of tenure of the young with their parents is unknown. Pentails foraged alone, perhaps in partially exclusive foraging domains within the area used by the group. Group life in pentails therefore is not likely to be related to shared foraging information or security from predators while active, because they avoid each other while foraging. This pentail social organization resembles that described for a tiny emballonurid bat, Rhynchonycteris naso (Bradbury and Vehrencamp 1976), in which adult members of a colony use individual solitary foraging beats, juveniles forage near the colony, and an adult male uses the
A single den tree formed the hub of pentail social life, and perhaps some special safety or other feature of the den site makes it a rare commodity in which a pentail family forms a nucleus. Because pentails are torpid and defenseless by day (Whittow and Gould 1976; Gould 1978, pers. obs.), they may require unusually well protected sites, such as hollow, high canopy branches with small entrance holes (perhaps from within the tree) that predators such as monkeys, martens, weasels, and snakes cannot enter or dismantle. In captivity P. lowii has the odd behavior of liking to sleep in glass jars (Lim 1967; Gould 1978). Gould (1978: 6) reported, “Despite an abundance of snug and quiet nest boxes as many as three pentail shrews crowded themselves into a single jar.” This mystifying behavior (the jars were clear, transparent glass) may indicate a preference for tight, smooth-walled narrow tubes such as branch hollows. Grouping also may have physiological advantages. If the diurnal torpor is an energy-saving mechanism (implying that pentails have an energy problem), then group huddling while inactive, as pentails do in captivity, may conserve heat and energy (Whittow and Gould 1976; Gould 1978).
territory formation and failure
The species of Tupaia differed in the cohesiveness of pairs that shared territories, as defined by the amount of recorded interaction. Tupaia minor and T. montana pairs spent the most time together, including some complete days of activity. These two species had the smallest home ranges (table 7.2) and were thus the most likely to be near each other by chance, but lesser treeshrew pairs often traveled together while insect foraging. The other three Tupaia had only brief contacts, sometimes daily, which consisted of male-initiated visits to females, within solitary foraging itineraries.
An intriguing behavioral question is how pairs of such solo-foraging and independent individuals as T. longipes make their extended boundaries so strongly concordant over as much as 1,000 m of borders. Kawamichi and Kawamichi (1979) suggested that pair members use scent marks to adjust their boundaries to those of their partners, a likely possibility. If true, I surmise that only the male fits his territory over the range
In T. gracilis and T. longipes I witnessed the formation of new territories by females that slowly pushed resident females aside and took over all or part of their space within the territories of resident males (F173, F86). This gradual encroachment is distinct from the all-or-none results of contests between mammals that fight physically for dominance. I suspect that these female-female interactions took place without much influence by the males: the male slender treeshrew visited the areas of each of the two females almost daily, and it seemed that he acquired two females by their spatial rearrangement, without changing his own. This was probably also the situation with T. longipes M64 on the territories of F56 and F86, but his boundaries during their interactions were unknown.
Despite the presence of clear territorial boundaries, the territoriality of Tupaia species has some flexibility. The Kawamichis saw the breakdown of territoriality at a large fruit tree:
From early February until mid-March, one large fig tree (Ficus dubia) provided an ample supply of ripe fruits. The sweet smell was perceived by us 250 m from the tree. Besides a pair … whose territories included the fig tree, all nine adjacent residents and seven other residents from the surrounding area came to the tree…. In addition, nine non-residents including six vagrants were also counted there…. The owners of the tree chased the visitors of the same sex. By mid-March the figs were eaten or rotten, and the residents subsequently confined themselves to their previous ranges. (1979: 392)
At Danum Valley bait (trap) sites had a similar effect of drawing some Tupaia into the territories of others. Prebaiting probably enhanced this behavior. The ranges of one pair of radio-tagged T. tana also seemed to be extended for a few days by some long daily journeys to a fruiting Polyalthia sumatrana that was signaled by noisy flocks of hornbills and other birds. The treeshrews could have detected the bird activity at this tree from afar. Possibly territory owners do not or cannot invest much energy in defending large fruit sources that vastly exceed their own needs.
ecological aspects of territoriality
The solitary foraging, long daily active period, and large daily movements of treeshrews show that their food resources are scattered, small, and hard to find. Because they use almost all of the time available in a day for foraging activity, there is not much leeway for more. Defense of feeding territories may sequester the food supply and allow treeshrews to feed within a smaller time and space than they would if conspecifics competed on the same feeding grounds. treeshrew territoriality is very likely the simple defense of adequate food resources for reproduction coupled with the sequestering of his mate by a male. That territories of T. glis in Singapore were one-tenth the size of those of the similar T. longipes in Sabah (Kawamichi and Kawamichi 1979), but the social system was identical
Unlike most other nonvolant mammals, treeshrews have no sexual dimorphism in body size (see table 2.2). This is consistent with a social system in which long-term monogamous pairs are the norm, effective sex ratio is not highly skewed, and territorial possession is not determined by dramatic physical dominance or fights (Kleiman 1977). The slow territorial displacements we registered do not suggest that territories are contested by deterministic battles in which the strongest or largest animal wins during a single contest. The energetically difficult life of treeshrews may give them little time for activities other than foraging, and absence of sexual dimorphism may also reflect strong energetic constraints on body size. A pair of solitary foragers on a common territory may be the most efficient use of both space and time for cryptic animals for whom sociality carries no feeding or predator escape advantages.
what some other shrews do
The social organizations of true shrews and elephant shrews illustrate the kinds of patterns found in other primitive mammals and give a perspective on those of treeshrews. The true shrews are the most energy limited of all mammals, and energetics overshadows their lives, but the two subfamilies, Soricinae (including most New World and many Old World genera, such as Sorex, Cryptotis, and Blarina) and Crocidurinae (including many Asian and tropical genera, such as Crocidura and Suncus), seem to have different life history characteristics, or strategies (reviewed in Churchfield 1990). Sorex species, where known, form exclusive territories as subadults or young adults, with no overlap between sexes or individuals during the winter, but in the summer breeding season males start to wander over female ranges, and offspring overlap with adults. Males seem to have no positive interactions with females or young apart from copulation. In the larger Blarina brevicauda territoriality in winter is exclusive between all individuals, but in the summer it is exclusive within the sexes, and males and females overlap. Cryptotis parva are gregarious and live in small colonies where adults share the same nest and home range. In several Crocidurines (Suncus and Crocidura species) there
These other small insectivorous mammals therefore have an array of social structures some of which are the same as those of tupaiids. Even such tiny, short-lived mammals as shrews express a wide spectrum of social organization, including solitary, monogamous, and group living species, and spatial organization that ranges from extensive overlap to territoriality between or within sexes. Advanced cognitive powers are not needed for expression of any of the mammalian social systems, which show great variability within families and even within genera. Tupaia species have a social system like that of some prosimians, but so do shorttailed shrews, agoutis, elephant shrews, and spiny rats. The spatial organization of mammals is plastic and changes to accommodate changing ecological conditions.