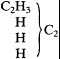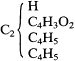Kolbe's "Merry Celestials"
The phrase in the section head ("hellgeborene heitere Joviskinder") derives from Otto Erdmann and designates in an avuncular and humorous way—but not favorably—the modernist followers of Kekulé in the chemical community.[1] Like Kolbe, Erdmann and many other older chemists were bewildered by the explosion of new ideas and distrusted their power to specify details of molecular architecture. It is a bitter irony that many of the best chemists who passed through Kolbe's lab were (or later became) structural organic chemists: in a semichro-nological list, we can name Griess, Claus, Crum Brown, Volhard, Graebe, Zaitsev, Menshutkin, Markovnikov, Armstrong, Meyer, Ost, Curtius, and Beckmann. Even Edward Frankland, one of Kolbe's oldest and best friends and whom he always considered in some sense a protégé, was distinctly structuralist by the time Kolbe arrived in Leipzig. All of these men had the highest regard for Kolbe, and he reciprocated that feeling. Several of them tried to persuade Kolbe that many structuralist theories were not very different from his ideas and that he ought to pay more attention to them. Some also remonstrated with him about his retention of the older equivalent weights long after nearly all of his colleagues had switched to atomic weights.
A discussion of Kolbe's views on chemical constitution and a comparison with structuralist ideas can be found in chapters 8 and 9. Kolbe's essential peculiarities were his absolute denial of direct
carbon-carbon bonds—the basis of what he called Kekulé's "chain theory"—and his conviction that there was a strict hierarchy of constituent radicals in every compound, with only one being the molecule's "fundamental radical" (Grundradikal or Stammradikal ). We have seen how Kolbe was able during his later Marburg years to more than hold his own in his encounters with the structuralists on a variety of fronts. However, as structural chemistry blossomed and flourished during the late 1860s, Kolbe began to lose pace with the field—as we have already seen in the last chapter as regards aromatic chemistry.
One example of this trend involves the question whether the four valences of carbon might be chemically distinct. Many structuralists, including Butlerov, Erlenmeyer, Crum Brown, and even Kekulé, thought for a few years that this was probable, for there were several cases of apparent isomerisms that could not otherwise be explained. The most notorious such case was the isomerism of "methyl gas" (CH3 -CH3 ) and "ethyl hydride" (CH3 CH2 -H), the first produced by electrolysis and the second by reduction of ethyl compounds, where the two indicated bonds were presumed to engage carbon valences of different chemical value. It was Frankland who discovered and defended this isomerism most vigorously, for the two compounds, although very similar themselves, seemed to result in quite distinct chloro derivatives.
In 1864 Carl Schorlemmer, an expatriate German working at Owens College Manchester, demonstrated the probable identity of methyl gas and ethyl hydride and argued that they formed an identical series of chloro derivatives. Schorlemmer's paper made a great impression, and little was said thereafter by structural chemists about differences between carbon valences. Frankland himself was largely convinced.[2] For two years he had been writing formulas using the reformed weights, and he was then on the verge of adopting Crum Brown's graphical formulas and becoming a structural chemist in the full sense. He wrote to Kolbe, asking him what he thought about Schorlemmer's work.
Kolbe was profoundly un convinced. He would only grant that Schorlemmer had demonstrated that both hydrocarbons yield the same ethyl chloride, still maintaining that "ethyl hydride" is a derivative of methane:
whereas "methyl" consists of two methyl radicals
![]()
This was was a departure for Kolbe, for it is the earliest indication that he had finally accepted the dimeric character of hydrocarbon "radicals" (we have seen that he conceded the importance of Wurtz' evidence for this thesis as early as 1855). To maintain his distinction between the hydrocarbons while simultaneously conceding the identity of their chlorinated derivative, he suggested to Frankland that there was a molecular rearrangement of "methyl" to the "ethyl hydride" constitution during the course of the reaction.[3]
To put it simply, from the time he first accepted the tetravalence of carbon (in 1858) to the end of his life, Kolbe assumed that the four radicals around a carbon atom were held with different degrees of affinity. This was also true for other atoms, he thought; the oxygens of sulfuric acid, the hydrogens of ammonia, and so on, were all chemically distinguishable.[4] After Schorlemmer's work, there was little evidence for this idea that most chemists found compelling. Kolbe, however, kept coming back to a single argument again and again; the fact, for instance, that methane can be monochlorinated proved to him that one of the hydrogens of methane is held less tightly than the others. Similarly, the existence of monoderivatives of benzene demonstrated conclusively, he thought, that the six hydrogens are chemically distinguishable, hence Kekulé's theory cannot be right.[5] The empirical failure of the assumption of chemically distinguishable valences, namely, the fact that it predicts numerous isomers none of which were ever found, was excused by Kolbe again and again by one of two gambits: molecular rearrangements or insufficient empirical experience. The structuralists' explanation for monoderivativization, that one hydrogen is randomly selected and the process stops after the first substitution, meant nothing to Kolbe.
These issues were brought to a head for Kolbe in 1866 and 1867 as a result of work on the base-catalyzed self-condensation of ethyl acetate performed independently by Anton Geuther at Jena and by Frankland and Duppa in London. Frankland's work arose out of his successful alkylations of esters using either zinc alkyls or sodium plus alkyl iodides—the final payoff from twelve years of trying to "ascend the homologous series of organic bodies" (see chap. 8). The diethyl substitution product in oxalic ester acquired the name "isoleucic acid,"
Et2 C(OH)CO2 H, because it was an isomer of the hydroxyacid derived from leucine; reduction of the hydroxyl group led to a branched-chain isomer of the straight-chain caproic acid, C5 H11 CO2 H. Substituting one and two ethyl groups into acetic ester yielded butyric and isocaproic acids directly.[6] In a remarkable paper published in 1866, Frank-land and Duppa announced the synthesis by a similar route of acetoacetic ester, as well as of ethyl- and diethylacetoacetic acid, (CH3 CO)Et2 CCO2 H. They found that these compounds decarboxylate in base to produce acetone and ethyl- and diethylacetone, respectively.[7]
It was during the course of this research program (in 1863-1866) that Frankland came over to structural ideas and to Crum Brown's graphical formulas. The details of his intellectual odyssey are not known, but one may presume that he found the modern ideas and formulations simpler and heuristically more valuable than the older ones. Frankland is a good model to compare with Kolbe because both men started from a similar set of views. No one understood Kolbe and his ideas better than Frankland, and no one was better situated than he to help Kolbe to follow along the same path that he had trod. He sent Kolbe an offprint of the culminating paper of this series.
Kolbe studied the paper with care and responded privately at length.[8] That Kolbe had truly invested sincere effort in understanding the paper is evident, not only from the fact that he was able to translate all of Frankland's formulas accurately into his own notational style but also because he suggested a reaction mechanism for acetoacetic ester synthesis that Frankland immediately recognized as superior to his own published conjecture.[9] But it is just as clear that Kolbe failed to understand the structure-theoretical principles upon which Frankland was reasoning. He objected to many of Frankland's condensed formulas because they seemed to posit groups of carbon atoms whose total combining capacity appeared to be short of the number required by tetravalence (for example, a group of two carbon atoms [C = 12] with a valence of six rather than eight, or four carbons with a valence of ten rather than sixteen). The structuralist interpretation of these formulas required subtracting from the sum of all the valences the number necessary to form carbon-carbon bonds, as Kekulé had discussed seven years earlier in the pages of his Lehrbuch der organischen Chemie . Kekulé's treatment was surely the clearest and most influential early exposition of the principles of structure theory, but Kolbe never read it, or at least not until many years had passed.[10]
Kolbe also disagreed with Frankland's formulation of the de-carboxylated compounds as derivatives of acetone. Rather, Kolbe averred, they were derivatives of methane, for example,
One could conceive of an ethylated or diethylated acetone, but that is a different compound, he argued, because it is derived from a different fundamental radical (Stammradikal ). Kolbe's systematic and elementary way of attempting gently to disabuse Frankland of his putative errors indicates that Kolbe had simply failed to follow Frankland's and Duppa's structure-theoretical exposition. He was also strongly opposed to the use of graphical formulas:
Frankly, I believe that all of these graphic representations are inappropriate for the times and are even dangerous, because they leave too much scope for the imagination, as for example happened with Kekulé: his imagination bolted with his understanding long ago. It is impossible, and will ever remain so, to arrive at a notion of the spatial arrangement of atoms. We must therefore also take care to avoid drawing a picture [of that putative arrangement] for ourselves, just as the Bible warns us from making a visual depiction of the Godhead.[11]
Kolbe laid all this out (without the metaphors) in a publication a year later. He wrote, "I expect to hear the objection which is repeatedly made to me orally," that his formulas are identical with Frankland's and Duppa's. "To be sure, they may appear so on superficial examination," but he and Frankland had chosen different fundamental radicals, and so it was clear that they were referring to isomeric and not identical compounds—just like, for example, ethyl acetate versus methyl propionate.[12] For Kolbe, choice of the base radical appears to have been crucial because of the presumed differences in carbon valences, but he did not make this assumption explicit.
Kolbe's friends and old students commiserated with each other. Frankland wrote to Crum Brown about this correspondence: "I am just now endeavoring to get Kolbe to express certain of his fundamental formulae graphically. We should then understand each other better." Crum Brown replied,
I quite agree with what you say of Kolbe. I worked with him for a summer session at Marburg. I was always able to explain any theoretical views of mine to him by first translating them (sometimes with a good deal of trouble) into his language, but I am quite sure he would fail to recognize his own ideas if translated into our language. For instance as to his HO . . . [C2 O2 ]O he certainly regards the HO as water, but he recog-
nizes the fact that there must be as many HO's at the beginning of the formula as there are O's outside the bracket at the end of it, & he points out (for the first time I believe) that [C2 O2 ] or CO is a group capable of combining with two equivalents & that in acetic acid it is combined with one equivalent (or atom) of C2 H3 methyl & one equivalent (or as we shd say 1/2 atom) of oxygen. To this he adds HO & as far as I can see the only difference between his view & ours is that he does not explain why. We say it is because the 1/2 atom of Oxygen in the HO is the other half of that which is combined with the CO. His obstinate opposition to O = 16 prevents his accepting such an explanation.[13]
Kolbe's article forced Frankland and Duppa to respond publicly. They praised Kolbe's proposed mechanism, agreeing that it was more probable than their suggestion, but they fulfilled Kolbe's prediction by arguing in great detail how the formulas they employed for the new compounds, both condensed and graphical, were entirely equivalent to his, providing that "Kolbe does not invest in his symbols some meaning that we cannot understand." They noted that the dividing up of a constitutional formula into radicals was a purely arbitrary operation, a matter of expository convenience and not ontology. Consequently, they were uncertain of the exact significance that Kolbe attached to his apparently important word Stammradikal . But even if there were a real difference in the compounds due to a difference in the "fundamental radical," they argued, this is certainly not analogous to the ethyl acetate versus methyl propionate case: the latter compounds have differences in the bonding order of the atoms in the molecule, whereas the Frankland-Duppa and the Kolbe formulas indicate the same atoms combined in apparently the same way.[14]
Not only Crum Brown, but also Graebe, Volhard, and other senior workers who passed through Kolbe's Marburg laboratory had to do the same sort of routine formula translations as Frankland did in order to converse (and argue) with the master. Referring to the same summer semester as Crum Brown had (1862), Graebe later wrote,
The radical theory as he developed it was at that time an excellent point of departure from which to understand structure theory, which was just then developing. It was only necessary to rewrite his formulas (at that time still being written in equivalents) into the new atomic weights, in order easily to understand the structural formulas.[15]
Volhard, too, did this sort of routine formula translation and became the first from Kolbe's lab actually to publish in atomic weights.[16] The same was true, of course, in Leipzig. Armstrong arrived there from Frankland's laboratory in 1867, and several Butlerov students passed
in and out during the early Leipzig years, especially A. M. Zaitsev and V. V. Markovnikov; all had absorbed structural ideas from their first mentors. Kolbe stated repeatedly, and all evidence from his students substantiates the claim, that he encouraged his students to follow their own ideas, to disagree and argue with him. A surprising number of publications by his students in Leipzig contain views with which he was known to disagree. At times this went so far that Kolbe attached long footnotes to student papers outlining his disagreement with them.[17]
Markovnikov's memoirs contain the most revealing anecdotes along these lines. Soon after his arrival in Leipzig in the fall of 1866, he wrote Butlerov (for whom he had served as assistant and lecturer in Kazan) that Kolbe was far from a martinet, that he positively enjoyed arguing with students, and that he (Markovnikov) had already succeeded in locking horns with him more than once. Moreover, Kolbe actually used Markovnikov to help him interpret modern formulas.[18] As it happened, Markovnikov's research project was related to the Frankland-Duppa material, for Markovnikov was attempting to show that Frankland and Duppa's product from the dimethylation of oxalic ester was identical to Staedeler's "acetonic acid" and that both had the structure of what could be more rationally named hydroxyisobutyric acid. This project was successful.[19]
Markovnikov related many years later that as he was writing up these results and preparing to leave Leipzig for Kazan (this must have been in the summer or early fall of 1867), he had one more tussle with his mentor. Kolbe had claimed that one of his formulas was wrong, but Markovnikov was emboldened by one of Kolbe's assistants who privately sided with him. He was invited into Kolbe's private office to discuss the matter.
The heart of the dispute was the old oxygen theory. "You don't understand me because you are not used to my formulas," said Kolbe; "I will express your thoughts in your own formulas." "Aha," I thought, "now, Herr Professor, you are mine." . . . He began to write, stopped halfway through the formula, thought a minute, then set the pencil down. "Ja, Sie haben Recht." Then he completed the formula and said once more, "Yes, yes, this is true; you are right," and somewhat confusedly began to explain something. I quickly retired, to spare the self-esteem of an honored teacher. A year later, I received from him a pamphlet on another of our disputed questions. In it he developed his theoretical ideas in detail, but now he wrote the weight of oxygen in the new way.[20]
It would be nice to know more details. What was the disputed point, and what was the formula? There is no particular warrant for the accuracy of this story, but the timing seems to fit. It was a little more
than a year after Markovnikov's departure that Kolbe sent colleagues offprints of his dissertation on hydrocarbons, which was the first time he published in the new atomic weights. On the other hand, Markovnikov was only one of several who were putting pressure on Kolbe at the time; Frankland's article in rebuttal to Kolbe's public response may have had more influence on him, at least regarding the atomic weight issue if not the more general formula question.[21]
The actual point of conversion for Kolbe from the older to the newer weights appears to have been the summer of 1868, just when Carl Graebe was beginning to pass the various stages in the habilitation procedure. Graebe had studied with Kolbe for a semester in Marburg, but had been most strongly influenced by the structuralist school, especially by Baeyer; his Habilitationsschrift was entirely based on the Kekulé benzene theory. The phrase "hellgeborene heitere Joviskinder " (quoted at the beginning of this chapter) was directed sarcastically to Graebe, but its author (Erdmann) and Kolbe both enthusiastically approved the habilitation. The fact that Graebe chose Kolbe's lab for habilitation, certainly with full knowledge of Kolbe's contrary theories, indicates once more that Kolbe was trusted implicitly (by those who knew him) to respect opposing points of view.[22]
In the event, Graebe was Privatdozent in Leipzig for only one semester, winter 1869/70. One of his auditors was Kolbe's student Ernst von Meyer, who was then in his fourth semester of study. In his memoirs, Meyer stated that he derived great profit from Graebe's instruction, absorbing and learning to appreciate the new structural chemistry. From then on, he added, he could read with full understanding articles in both the older and the newer styles and had no difficulty in translating one into the other. Although he agreed with Kolbe's criticism of the sloppy and conjectural character of much modern chemistry, Meyer respected and valued structure theory, a point that he stressed in correspondence and that emerges clearly from his historical writing.[23]