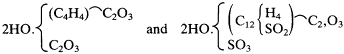Chemical Editor
At Vieweg Verlag, Kolbe set to work energetically at his editorial tasks. One of his earliest decisions was notational. There was at that time disagreement on certain important points, and there had been several recent international shifts in formula conventions. Above all, should one take H = 1, H = 2, or H = 2, in other words, should one notate benzoyl chloride, for example, as C14 H10 O2 Cl2 (Berzelius' notation until 1826 and Liebig's until 1844), C14 H5 O2C l (Berzelius' and Wöhler's notation since 1826), or C14 H5 O2 Cl (Liebig's notation since 1844)? To Wöhler, Kolbe expressed a preference for H = 2. Wöhler agreed but remarked that Kolbe had used H = 2 in his dissertation, and he noted that there are problems of consistency in the Handwör-terbuch as a whole since the first volumes, following Liebig's preference of the 1830s and early 1840s, had used H = 1. Wöhler suggested Kolbe check with Liebig before settling the point. Kolbe did this, and Liebig agreed with the proposal to use barred letters to remove any possible ambiguity.[44] Kolbe's pattern in his own articles at this time was to use barred letters for German publications and the unbarred (H = 2) letters in England, as had become accepted there. The English system was soon thereafter generally adopted in Europe. This system
is notationally equivalent to taking H = 1, C = 6, O = 8, Cl = 35, and so on, which are the conventional equivalents that became so universally popular during the late 1840s and 1850s.[45]
Kolbe discussed these and other notational issues in articles written for the Handwörterbuch sometime in the middle of 1848. He explained his preference for the barred symbols, while sharply rebuking the French for maintaining Dumas' hybrid system of H = 1, C = 6, and O = 16 with four-volume organic formulas. This was, he thought, an "irresponsible" system that only increased the existing confusion, and was defended only to attempt to maintain an "imagined law" based on "vague hypotheses."[46] This, his first strong public derogation of the French, may have been partially motivated by feelings aroused by the violence of the February revolution in Paris and the consequent upheavals in Germany of the "March days." Kolbe's moderate liberalism did not extend to toleration of insurrection or anarchy.
In this article Kolbe also discussed how the notation of chemical formulas
. . . attains great importance by offering us a means of representing with greater sharpness and precision the different conceptions concerning the chemical constitution of a compound, solely by the various ways of grouping a few symbols, thereby simultaneously expressing a summary of ideas that can be reproduced so briefly in no other manner.[47]
So, for example, the constitution of acetic acid might be represented by the various formulas C+HO, HO.C4 H3 ,O3 , or ![]() . Whereas the first is little more than an empirical formula, the second accounts for the acidic and some of the electrochemical properties of the substance. The last, however, also accounts for the evidence supporting a copula formula: hydrogen of the methyl copula can be substituted by halogen without breaking up the structure of the radical, or the methyl copula can even be transferred to another radical entirely, as, for example, in the transformation of acetyl derivatives into methyl cyanide or cacodyl compounds.
. Whereas the first is little more than an empirical formula, the second accounts for the acidic and some of the electrochemical properties of the substance. The last, however, also accounts for the evidence supporting a copula formula: hydrogen of the methyl copula can be substituted by halogen without breaking up the structure of the radical, or the methyl copula can even be transferred to another radical entirely, as, for example, in the transformation of acetyl derivatives into methyl cyanide or cacodyl compounds.
Two months later, Kolbe provided even more detail on his ideas of the constitution of acetic acid. The radical of acetic acid is methyl copulated to "oxatyl," C2 , the resulting hydrocarbon radical to be called "acetyl," ![]() . Oxatyl was "the exclusive point of attack for the affinities of those elements," such as oxygen, that formed the various compounds of acetyl. For instance, the first stage of oxidation of acetyl was aldehyde
. Oxatyl was "the exclusive point of attack for the affinities of those elements," such as oxygen, that formed the various compounds of acetyl. For instance, the first stage of oxidation of acetyl was aldehyde ![]() , the third stage being acetic acid
, the third stage being acetic acid ![]() . In other words, reagents add only to oxatyl, and conversely, oxatyl undergoes only addition reactions. Substitution
. In other words, reagents add only to oxatyl, and conversely, oxatyl undergoes only addition reactions. Substitution
reactions (i.e., those that occur without major alteration of chemical properties) could take place only in the copula: for example, ![]() . Organic radicals could now be subsumed into two distinct groups: hydrocarbon radicals and conjugate radicals such as those of the acetyl class, which could then form "secondary" conjugate radicals (those that have undergone substitutions).[48]
. Organic radicals could now be subsumed into two distinct groups: hydrocarbon radicals and conjugate radicals such as those of the acetyl class, which could then form "secondary" conjugate radicals (those that have undergone substitutions).[48]
In this fashion, Kolbe had schematically resolved the acetic acid molecule one step further than ever before by distinguishing and focusing on what has become known as the carbonyl carbon atom. He had also created a theory of greater generality and wider application: radicals fully analogous to oxatyl could be formed from such elements as arsenic and sulfur, forming the constitutional basis for cacodyl and sulfonyl compounds. In this article, Kolbe thus altered his interpretation of the organic acids from formulas based on copulated oxalic acid to a more generalized concept using copulated oxide hydrates of carbon and other atoms—a progressive and fruitful shift.[49]
The buckle symbol, ![]() , Kolbe noted, had been proposed to him by Friedrich Otto (1809-1870), a professor of technical and pharmaceutical chemistry at the Collegium Carolinum, as a convenient way to indicate the special form of chemical bonding in copulated compounds. As for periods and commas, Kolbe defined them as equivalent in denotation; from this time forward, however, he tended to use the period for indicating the attachment of "basic water" to the remainder of an organic formula and the comma for separating oxygen from hydrocarbon moieties—though he sometimes omitted it. Parentheses presumably further emphasized the integral character of certain radicals. The option of dispensing with all of these punctuation marks and relying on simple juxtaposition of grouped letters, as he did, for instance, with C and H in hydrocarbon radicals, apparently did not sufficiently satisfy Kolbe's electrochemical instincts.
, Kolbe noted, had been proposed to him by Friedrich Otto (1809-1870), a professor of technical and pharmaceutical chemistry at the Collegium Carolinum, as a convenient way to indicate the special form of chemical bonding in copulated compounds. As for periods and commas, Kolbe defined them as equivalent in denotation; from this time forward, however, he tended to use the period for indicating the attachment of "basic water" to the remainder of an organic formula and the comma for separating oxygen from hydrocarbon moieties—though he sometimes omitted it. Parentheses presumably further emphasized the integral character of certain radicals. The option of dispensing with all of these punctuation marks and relying on simple juxtaposition of grouped letters, as he did, for instance, with C and H in hydrocarbon radicals, apparently did not sufficiently satisfy Kolbe's electrochemical instincts.
In these articles, which contain his most extended descriptions of the notational conventions that he followed with minor variation for the rest of his life, Kolbe made few of these details explicit. What does emerge clearly, though, is his conviction that carefully constructed formulas are extraordinarily useful semiotic and heuristic devices for the development and communication of theoretical ideas. In fact, to a degree perhaps unmatched by any other chemist of his day, Kolbe's notation cannot be separated from his theories. This is true because every formula that Kolbe wrote was a deliberate theoretical statement, namely, an assertion regarding the constitution of the molecule being discussed.
The Handwörterbuch project, barely alive for so many years, took off immediately under Kolbe's leadership. He solicited scores of arti-
cles from competent authorities and rode herd on them until they delivered; he then edited their contributions. He wrote a number of significant articles himself, among them Formeln, chemische, Formyl, Gepaarte Verbindungen, Kakodyl , and Kohlenwasserstoffe , and he revised articles on Acetyl, Aethyldithionsäure , and Benzoësäure . By 1851, the third and fourth volumes were complete through letter L, as well as a supplement volume. More progress was made in four years under Kolbe's leadership than had been made in the previous fifteen years. Liebig, Wöhler, and Poggendorff remained the "Herausgeber, " with Kolbe as "Redakteur. "
Kolbe also accepted another literary assignment early in his tenure in Braunschweig, which would lead to an enormous literary opportunity—and burden—occupying him for the rest of his life. Someone, probably either Friedrich Otto or Eduard Vieweg himself, conceived the idea that it would be a valuable (or profitable) undertaking to publish a German version of Thomas Graham's popular English textbook of chemistry. The first German edition (1840-1843) was a simple translation by Otto. For the second edition, conceived as a substantial rewrite of the original, Otto wrote the first volume, on general, physical, and theoretical chemistry (1845-1847), and Kolbe was asked if he would edit the organic portion of the text. On Christmas day 1847 we find Bunsen replying to Kolbe's request for advice on this point; Bunsen urged him to accept Vieweg's offer, but cautioned him not to overcommit on too many projects—sage counsel![50] We can only conclude that he must have worked at least occasionally on this project during the next three and a half years, since by the time of his arrival in Marburg, he had a manuscript of at least a portion of it, which he put to good use in preparing his lectures. But the manuscript went through several incarnations before it actually began to appear in fascicles in 1854.[51] By then, Kolbe had long been referring to it as "meine Organische. " It was no longer a translation, nor even a translated revision of Graham's work, but a detailed advanced organic chemistry text written by Kolbe ab ovo —although still published with a second title page giving the Graham-Otto imprimatur.
Frankland mentions, and we are told by biographers, that Kolbe had occasion to work in Varrentrapp's laboratory during his years in Braunschweig. No evidence for this is apparent from Kolbe's publications or letters, and fifteen years later he explicitly stated to Frankland that he had had no opportunity for laboratory work in Braunschweig.[52] He published only one article containing original experimental work during this period, and that work appears to have been done when Kolbe returned to Bunsen's lab in Marburg for six weeks or so in the summer recess of 1848 and again for "a week or so" in the summer re-
cess of 1849, as Frankland reported. He returned for a third time in the summer of 1850, spending a month in Marburg, Giessen, Frankfurt, and Heidelberg.[53] Frankland related: "He was then in his prime, full of enthusiasm for organic chemistry and earnestly hoping for a professorship, which would afford him the much desired opportunity for work."[54]
The work done by Kolbe on the first of these occasions was particularly fruitful. On the first day of August 1848, Kolbe wrote Frankland from Marburg, hoping that Frankland would arrive before he had to return to Braunschweig. He was working on the electrolysis of malonic and acetic acids. Six days later, he reported to Vieweg, "My chemical work, for which Bunsen's laboratory offers in fullest measure all the necessary equipment, has already achieved the geatest success, so that within a short time I will have reached the goal I had set for myself. Bunsen himself shows the greatest interest in my experiments. . . ."[55] A paper summarizing the results of this work was sent early the following year to Liebig for the Annalen and to London for the Journal of the Chemical Society .[56] Liebig thought the work "magnificent" and agreed with Kolbe's conclusions. "It has been a long time," he wrote, "since I have read an article that has excited me as much as yours has. The thoughts are as lovely as the methods, and the development is masterly." He had dabbled along similar lines himself, he said, but without this kind of success, and was happy to leave the field entirely to Kolbe.[57]
In this paper, Kolbe succeeded in sorting out many of the difficult details of the electrolysis reaction he had been working on since London. The hydrocarbon radical from decarboxylated potassium valerate he named "valyl"; he guessed that it must be the radical of the still unknown butyl alcohol. Even more important, acetic acid, barely mentioned in the London paper, now gave clean results: hydrogen, carbon dioxide, and methyl were all among the products, and the methyl gas proved chemically identical to that which he and Frankland had prepared from ethyl cyanide. (Kolbe's "radicals" are now considered to be their respective dimers, octane and ethane.) Whereas in 1844 he had fully synthesized acetic acid, this most fundamental organic substance, he had now successfully analyzed it into its component parts. They were the radicals predicted by his Berzelian theory.
This success gave Kolbe the impetus to write a long critical review of recent research on organic radicals, together with the various theories that were then contending to explain those results. Although he was at work on this review as early as March 1850, it was not until that fall that he completed and submitted the article, both to the Annalen and to the Journal of the Chemical Society . Hofmann was
Foreign Secretary to the Chemical Society, and in a reply to Kolbe, he offered either to translate Kolbe's article or to have it translated under his direction.[58]
Kolbe began by summarizing the disputes of the 1830s and 1840s between the (principally German) Berzelians and the French substitutionists, centering on the constitution of acetic acid and its derivatives. It was now beyond question that substitution does take place without altering the fundamental chemical properties of the substance and that organic radicals are by no means inviolable, as Berzelius had initially wanted to maintain. But Kolbe insisted that this by no means destroyed, or even substantially weakened, the electrochemical theory. One need only hypothesize further structure in one's radicals to provide a fully adequate theoretical explanation for all the new reactions.
For acetic acid, this meant that it was no longer sufficient to suppose a single integral "acetyl" radical, C4 H3 , combined with three equivalents of oxygen to form the anhydrous acid, as Liebig had been doing for the past decade. Kolbe's new formula was ![]() , in which the oxatyl group C2 "presents the exclusive point of attack for the powers of affinity of oxygen, chlorine, &c.," as described in his Handwörterbuch article of 1848. This in turn suggested a new mechanism for the reaction in which alcohol is oxidized to aidehyde:
, in which the oxatyl group C2 "presents the exclusive point of attack for the powers of affinity of oxygen, chlorine, &c.," as described in his Handwörterbuch article of 1848. This in turn suggested a new mechanism for the reaction in which alcohol is oxidized to aidehyde:
![]()
Kolbe thought that the oxygen must induce the ethyl radical to split into methyl plus C2 H2 , whereupon the two hydrogen equivalents in the latter moiety are captured by the extra oxygen, while the existing oxygen in the compound remains. Oxidation of aldehyde proceeds then by the direct addition of two more oxygens to the C2 . Substitution can complicate the notation of conjugate radicals, such as sulfobenzoic acid:[59]
![]()
In addition to the conjugate radicals found in copulated compounds, Kolbe named his second class of hydrocarbon radicals, possessing different chemical properties, the "ether" or "alcohol" radicals, which provided an idea of the constitutions of the homologous ethers and alcohols and their derivatives. A third class of radicals, new to this article, was the "homologizing hydrocarbon radicals," Cn Hn , as in succinic or adipic acids:
![]()
For the first time, we see here Kolbe admitting the possibility of dibasic organic acids, and he conceded that it might be necessary to reformulate succinic and sulfobenzoic acids as
where the numbers of atoms are simply doubled and rearranged in the first formula and another sulfate group is added in the second.[60] A curious aspect to this particular manipulation is that the second formula does not maintain the correct atomic ratios.
It is unclear precisely how Kolbe assigned some of his structures, in particular how he knew which compound was in the ether and which in the conjugate radical series. For example, according to Kolbe's theory, methane and ethane are actually methyl hydride and ethyl hydride, whose radicals are in the ether series. The chemically similar chlorinated derivatives perchloroethylene and perchloroethane are, however, conjugate radicals:[61]
![]()
To be sure, Kolbe had shown six years earlier that one could transform perchloroethylene into chloroacetic acid, justifying the first formula. He had also, however, shown how to convert methyl hydride to methyl chloride to methyl cyanide to acetic acid, which ought to have suggested that the methyl in methyl hydride is also a conjugate radical.
Another weakness of Kolbe's position, and one that he recognized, was the uncertain status of electrochemical-dualist precepts in his theory. Although his organic electrolyses appeared to demonstrate anew the general validity of electrochemical ideas, his specific predictions of the outcome of the experiments not infrequently failed verification.[62] He conceded that in many organic substances, it even seems impossible to determine which elements are positive and which are negative, such as carbon and nitrogen in cyanogen or carbon and hydrogen in hydrocarbons. Perhaps hydrogen's electrochemical properties are different in organic versus inorganic compounds—after all, hydrogen certainly has different properties in the normal gaseous versus the nascent state. So might chlorine have a less negative character in organic compounds than in its natural condition. These, he admitted, must be seen as conjectures regarding yet unsolved difficulties.[63]
In any case, Kolbe had developed here a detailed electrochemical radical theory that he was proud to compare with the type theories of the French. His view was that Dumas, Laurent, and Gerhardt had rushed to discard Berzelius' theoretical edifice on the basis of a single new phenomenon—substitution. This was overly hasty, to say the least, since all one needed to do was discard the now untenable belief in the immutability of radicals in order to resurrect the time-tested Berzelian theory. He concluded
It would be ridiculous to allow a single fact, difficult of explanation, to induce us to throw aside at once a theory which has served us for so long a period as a trustworthy guide in the difficult field of organic chemistry, and has preserved us most securely from the errors of a code of laws like that which has been laid down by Laurent and Gerhardt—unless we had some better theory to substitute for it. . . . [C]hemistry is indeed something better than a mere arithmetic problem, into which Laurent and Gerhardt endeavor to convert it.[64]
In many respects, this was an impressive performance. The article is detailed, well documented, and (aside from some nationalistic aspersions in the conclusion) a fair-minded summary of much recent research in organic chemistry. And in one respect at least, it was revolutionary. Kolbe here adumbrated for the first time a concept that would prove central in the future development of the science, what modern organic chemists refer to as functional groups , and he began the process of locating functionality on specific parts of the "constitutions" or structures of molecules. It was Berzelius who first suggested that acetic acid could be considered as schematically dissectible into hydrocarbon and oxycarbon moieties; it was Kolbe who generalized that notion and drew out its implications. Kolbe showed that it is at the "oxatyl" carbon (in modern vocabulary, the carbonyl carbon) where the chemical functionality of the molecule is concentrated. This thesis was amply supported by dozens of reactions of acetyl derivatives and other methyl compounds. He attempted the same thing with all of the scores of compounds discussed in his paper.
But it was precisely in this respect where the greatest weakness of the paper lies. Kolbe aggressively followed the same pattern of identifying functionality and its location within the molecule that proved so successful for acetic acid, even for those much more numerous cases where little empirical data existed from which to conclude such details. His paper is full of formulas suggesting specific details of constitution and implying predictions of chemical behavior that were unsupported or unexamined in 1850. Along the same lines, he continued the prolif-
eration of notational symbols that were poorly defined or even undefined. Berzelius had used periods or commas, while others among his contemporaries used parentheses and brackets, but Kolbe's pen was exceedingly restless with regard to the density of such symbols, and he added single and double buckle symbols to identify conjugate and homologizing hydrocarbon radicals. This often produced quite complex formulas (some examples of which have been cited) to which might be attached various empirical interpretations or predictions. For psychological and structural reasons, he could not write a formula without deliberately suggesting these interpretations and predictions.
For instance, the implication of his formulas for dibasic sulfobenzoic or succinic acids (as cited earlier) suggests that the two acid functions of each molecule are not chemically the same. It was eventually determined that in one case, this suggestion was correct, while in the other case it was not. More damaging and more to the point, in neither case did he have any evidence on which to judge the issue. Kolbe's readers had the right to expect the verbal and symbolic distinctions between alcohol and conjugate radicals to match consistent differences between the chemical behavior of those radicals, such as their replaceability with halogen atoms, but he simply failed to pursue this evidence. As a final example, the formulas for the chloroethylenes and chloroethanes cited above imply chemical nonequivalence of both the carbon atoms and the chlorine atoms within these molecules, but there was at that time no evidence for either equivalence or nonequivalence.