Chapter Nine
Diseases of the
Cardiac Valves
The structure of the four cardiac valves is described in chapter 1. To recapitulate their function, the two inflow (atrioventricular) valves—the tricuspid valve on the right side of the heart and the mitral valve on the left—shut to keep blood from backing up into the atria during contraction of the ventricles (systole) and open wide during diastole to permit blood to enter the ventricles from the atria. The two outflow valves—the semilunar valves—separate the ventricles from the large arterial trunks: during diastole the left-sided aortic valve closes off the aorta from the left ventricle, and the right-sided pulmonary valve closes off the right ventricle from the pulmonary artery.
When functioning normally, each valve in the open position permits blood to flow unimpeded through the orifice it protects; to do so, it has to be completely open. In its closed position each valve has to seal the orifice tightly, so as to withstand the pressure difference between the cardiac chambers or great vessels. Thus when a cardiac valve malfunctions, blood may not flow freely through the open orifice or may leak back when the orifice is closed. The former abnormality is called stenosis (narrowing) of a valve, the latter regurgitation or insufficiency .
Both stenosis and regurgitation of cardiac valves have to be considerable to affect the function of the heart. Mild or even moderate stenosis has only a minor effect on the circulation, well within the capability of the heart to adapt. Significant stenosis is usually present
when the valve orifice is reduced to less than one-third of its normal size. The narrowed valve then produces resistance to blood flow, which causes pressure to rise in the chamber behind the valve. The consequence of regurgitation is backflow of blood through an incompetent valve; that is, the unidirectional flow of blood becomes bidirectional. Both stenosis and regurgitation increase the workload of the cardiac ventricles.
Rheumatic Fever
An acute disease involving many structures in the body, rheumatic fever particularly afflicts the joints and the heart. Whereas rheumatic disease of the joints heals without aftereffects, involvement of the heart may produce permanent damage to the heart valves; that damage is sometimes immediately apparent, but it may also go undetected for many years. Attacks of rheumatic fever vary in severity; it may manifest itself as an acute, serious disease associated with high fever or appear as minor swelling and pain in the joints. Rheumatic fever is a disease of childhood and in most cases affects persons between the ages of four and twenty.
Though resembling an infectious disease, rheumatic fever is not caused directly by a microorganism. Rather, the body reacts to certain strains of streptococcus (a common bacterium responsible for many different infections, including sore throat) in persons hypersensitive to the microorganism (a process akin to allergy).
The epidemiology of rheumatic fever is one of the more interesting phenomena in contemporary medicine. The prevalence of the disease was high in the United States and other Western countries until the 1950s. Since then its incidence has declined steadily; the cause of this shift is not well understood. By contrast, there has been a dramatic increase in the incidence (or perhaps recognition) of rheumatic fever in third-world countries. As a consequence, rheumatic fever is rarely encountered in the developed countries, whereas in the developing countries of Latin America and Africa and in India it has become the principal cause of heart disease.
A typical attack of rheumatic fever is disabling, producing painful swelling of the large joints—the knees, elbows, and hips—usually migrating from joint to joint and affecting only one at a
time. It usually lasts one to four weeks but occasionally persists for months. Involvement of the heart is common but, more often than not, inconspicuous and difficult to detect. Other organs may also be involved, such as the lungs, kidneys, and brain (producing chorea, or uncoordinated movements).
Involvement of the heart appears as carditis (also referred to as pancarditis), which consists of rheumatic changes in the three layers of the heart—the endocardium (valves), the myocardium (heart muscle), and the pericardium. Although carditis usually does not affect the function of the heart or produce symptoms, it occasionally brings on serious, even fatal, conditions, such as heart failure, severe incompetence of valves, or large pericardial effusion. Characteristically, changes in the myocardium and pericardium heal without any aftereffects. Rheumatic disease of the valves, however, produces small, wartlike lesions that often initiate a chronic process leading after many years to serious valve disease. The principal damage to heart valves occurs as a result of the healing and scarring of the acute changes. Stenosis of the valve is caused by adhesion between leaflets, incompetence by shrinking of the scarred valve. Thus chronic valve disease is not a direct continuation of the acute disease but usually appears after a long period of apparent complete recovery.
Rheumatic fever has a tendency to recur: a child hypersensitive to streptococcus may suffer an attack each time it is infected. Hence the standard treatment is prophylactic administration of an antibiotic (usually penicillin) to prevent streptococcal infection for many years after the first attack. Recurrence of rheumatic fever increases the severity of valvular disease. In the developing countries, where penicillin prophylaxis is difficult to enforce, children may have yearly attacks of rheumatic fever and develop serious valvular disease at a young age. Thus, though in most cases rheumatic fever is a self-limiting disease, its effect on the cardiac valves may produce disabling heart disease decades later.
Sequelae of Chronic Valvular Disease
Chronic valvular disease involves permanent deformity of a cardiac valve or its neighboring structure. The defect may be congenital (present since birth) or acquired during life in a variety of ways.
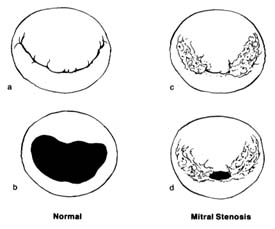
Figure 31. The mitral valve as seen from the left atrium. (a) Normal mitral valve, closed position,
showing two leaflets, one large and one small, covering the orifice completely. (b) Normal mitral
valve, open position. (c) Mitral stenosis, closed position, showing the valve with its peripheral
portion joined together at the commissures; the central opening still can close completely in
systole. (d) Mitral stenosis, open position; during diastole only a small central opening
permits blood flow, causing serious obstruction.
Stenosis of a valve, unless congenital, is always the result of a slow process, taking years or decades to reach the point of compromising heart function. Incompetence of a valve can develop abruptly or gradually: it may be caused by a disease of the valve itself, of the ring to which it is attached, or of the auxiliary structures supporting closure of the valve. The two left-sided valves—the mitral and aortic valves—are exposed to pressure several times higher than that exerted on the right-sided valves. The wear and tear on the valves as well as the fact that most cardiac diseases predominantly affect the left ventricle make these two valves more vulnerable. Furthermore, strain on the left ventricle from overload in valvular disease has more serious implications than does strain on the right ventricle.
Stenosis of the mitral valve (fig. 31), when significant, produces resistance to the blood flow during diastole from the left atrium to
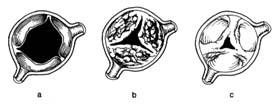
Figure 32. The aortic valve in its open position. (a) Normal valve.
(b) Aortic stenosis caused by calcium deposition producing stiffening
of the leaflets; the mobility of the valve is limited even though the
commissures are free. (c) Aortic stenosis caused by joining together of
the peripheral parts of its commissures, similar to mitral stenosis.
the left ventricle. To overcome the resistance and squeeze blood through the narrowed orifice, pressure in the left atrium has to rise (typically to 25 mm Hg instead of the normal 10 mm Hg), whereas the diastolic pressure in the left ventricle, normally identical with that in the atrium, remains unchanged. Mitral stenosis thus produces a pressure gradient across the valve, the magnitude of which depends on the severity of stenosis. High pressure in the left atrium makes the pressure rise in the pulmonary blood vessels, leading to pulmonary hypertension, which in turn overloads the right ventricle and may cause its failure. This mechanical dam at the mitral orifice has an effect on circulation through the lungs similar to that of left ventricular failure (see chap. 5).
Stenosis of the aortic valve (fig. 32) causes resistance of the ejection of blood into the aorta during ventricular systole, which has to be overcome by increased pressure in the left ventricle. Normally during systolic ejection pressures in the left ventricle and the aorta are identical. Aortic stenosis causes a pressure gradient across the aortic valve. For example, in severe aortic stenosis the pressure needed to eject blood into the aorta (which itself has a pressure of 120 mm Hg) may be as high as 240 mm Hg (a gradient of 120 mm Hg), doubling the systolic workload of the left ventricle and leading to its hypertrophy.
In mitral regurgitation the left ventricle, while ejecting blood into the aorta, also pumps some blood back into the left atrium. If the volume of backflow is appreciable—sometimes equal to the
forward flow into the aorta—the left ventricular workload is significantly increased.
In aortic regurgitation the incompetent aortic valve cannot keep blood from being sucked back into the left ventricle during diastole. In severe cases the amount of blood ejected by the left ventricle may be double or triple the normal cardiac output so as to compensate for the backflow, resulting in volume overload and hypertrophy.
In general, then, the effect of valvular disease on the heart is an increase in workload. In contrast to the cardiac consequences of coronary-artery disease, where the heart muscle is damaged and weakened, increased workload produces compensatory hypertrophy of the muscle of the affected ventricle. Thus the cardiac pump becomes stronger than normal, permitting the affected person to lead a normal life for many years. Disability develops late, when hypertrophy of the myocardium can no longer cope with the high workload. Valvular disease is the ideal target for surgical removal of the cause of overload. Not only can surgery correct the disabling symptoms and manifestations of heart failure in such cases, but it may lead to regression of cardiac hypertrophy as well.
The notion of dilating a stenotic mitral valve was expressed as early as 1900, though the means for doing so were not then available. In 1948 surgical technique reached the point where mitral valvotomy could be successfully performed. Throughout the 1950s stenotic valves were dilated by placing fingers or instruments into the beating heart. Mitral stenosis was the most frequent target for so-called closed-heart surgery. Closed mitral valvotomy produced great successes—even today the operation is performed on selected younger patients. (Operations on stenotic aortic and pulmonary valves were less successful and have been abandoned in favor of open-heart repair.)
But during closed operations on a beating heart no actual repair of incompetent valves was possible. The next breakthroughs in valvular surgery came with the introduction of the pump-oxygenator, which made open-heart surgery possible, and then the development of functioning prosthetic valves.
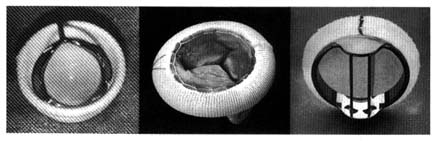
The first successful prosthesis consisted of a plastic ball within a metal cage (fig. 33, left). With the heart stopped, its functions temporarily assumed by the pump-oxygenator, the damaged valve
Figure 33. Examples of prosthetic cardiac valves. Left: Prototype mechanical ball
valve introduced in the early 1960s. Center: Biological valve taken from a pig's
left ventricle mounted on a specially designed ring.
Right: A mechanical tilting-disk valve.
was removed and the prosthetic valve sutured into the aortic orifice. Once the heart was restarted, the ball was propelled to the top of the cage by left ventricular contraction, allowing the blood to enter the aorta by passing around it. Ventricular relaxation pulled the ball back into the base, sealing the orifice. A similar ball valve was used to replace faulty mitral valves, with the cage inside the ventricle: ventricular relaxation pulled in the ball and opened the valve, permitting blood to flow from the atrium to the ventricle. Systole forced the ball back to its base, closing the mitral orifice.
Prototype ball valves worked well, with some patients surviving for more than 20 years. Yet the many technical problems and complications spurred the development of new devices. A new approach was introduced a decade after the ball valves, namely the use of biological valves, consisting of human or other animal tissue. The most successful and widely used biological valve is the aortic valve of a pig (porcine heterograft), mounted into a special frame that can be sutured into the mitral or aortic orifice (fig. 33, center). This three-leaflet valve is an ideal substitute for the aortic valve, but it also works well in the mitral position. Modifications of ball valves led to improvements that reduced some of the untoward consequences of the earlier designs. Other prosthetic valves now widely used are fitted with hinged discs to control bloodflow (fig. 33, right).
The major disadvantage of prosthetic valves is that as foreign bodies they are a prime site for the formation of blood clots. These clots may interfere with valvular function, but more often they
represent a potential source of emboli, which can cause stroke or other complications. Hence patients with prosthetic valves must remain on anticoagulant therapy throughout their lives. Biological valves greatly reduce the risk of thrombus formation; here anticoagulant treatment is administered only in selected cases. The disadvantage of biological valves, however, is their low durability, for the valve may stiffen and even calcify. The average duration of their normal function is seven to ten years, after which replacement is usually necessary. In children and young adults the process of biological-valve deterioration is greatly accelerated, so that their use in this age group is avoided.
Valvular surgery represents one of the most important advances in the treatment of heart disease, yet there are still many unsolved problems. None of the presently available replacement valves can function as efficiently as the normal natal valve. In addition to clot formation, a number of other complications may develop after successful surgery. And the risk of surgery is not negligible: surgical mortality is estimated at 5–10 percent and may be even greater in high-risk patients. Consequently, deciding the optimal time for valvular surgery is often difficult. Patients in heart failure or seriously disabled by a valvular disease are prime candidates for surgery. Those whose symptoms are still mild enough to allow a reasonably active life may best manage with nonsurgical medical treatment. Early operations in asymptomatic patients aimed at preventing future problems are inadvisable except in special circumstances. In considering candidates for valvular surgery, the physician usually takes into account the patient's occupation and life-style as well as the prognoses with and without surgery.
Clinical Features of Valvular Disease
Mitral Stenosis
Stenosis of the mitral valve is always the aftereffect of acute rheumatic fever. It affects primarily women (60–75 percent of all cases). It takes years for the gradual scarring process following the initial lesions of rheumatic fever to produce mitral stenosis. The interval between the attack (usually in childhood) and the development of symptoms varies widely. One factor is the severity of the attack of rheumatic fever. Furthermore, recurrent attacks lead to more-rapid
scarring of the valve. On average, mitral stenosis can first be detected 10 years after the initial attack; significant symptoms may not develop for another 10 to 30 years.
As described in chapter 1, the mitral valve consists of two leaflets separated by two commissures. The initial, rather minor damage to the valve during acute rheumatic fever consists of small lesions located usually at the edges of the leaflet. The healing and scarring process leads to the formation of adhesions between the leaflets, sealing the outer parts of the commissures, so that only the center part of the valve can open during diastole (see fig. 31, p. 129). The normal opening of the mitral orifice during diastole measures 4–6 cm2 . Only when the sealing process reduces the size of the orifice to less than 2 cm2 does the first effect of stenosis develop: the smooth flow of blood through the orifice becomes turbulent, giving rise to a murmur, which the examining physician can detect as evidence of mitral stenosis. However, at this point there is not enough resistance to flow to produce any effect on the circulation. Only further progression of the sealing process leads to the damming up of the orifice, raising the pressure in the left atrium. Usually when the opening is reduced to about 1.5 cm2 , the patient begins to experience shortness of breath with activity. At this stage (mild mitral stenosis) disability may be minimal, merely requiring the patient to curtail strenuous activities. Sometimes postrheumatic progression stops before symptoms develop, and the patient may go through life without any serious consequences of the disease, in some cases even remaining unaware of its existence. Further progression of mitral stenosis, leaving an orifice of 1 cm2 (moderate mitral stenosis), may produce more-pronounced symptoms and disability; furthermore, complications often develop. Severe mitral stenosis (with an orifice of 0.5–0.7 cm2 ) produces serious disability—congestive heart failure and, in some patients, severe pulmonary hypertension.
Mitral stenosis differs from the other valvular diseases in that the mechanical obstacle to flow occurs before the blood enters the left ventricle; hence that important cardiac chamber is spared increased workload. Yet the blood dammed in the left atrium produces an effect on the pulmonary circulation similar to failure of the left ventricle. Consequently, shortness of breath resulting in disability develops early, as soon as mitral stenosis becomes mild or moderate, whereas in the other diseases dyspnea develops late, only
when the hypertrophied left ventricle can no longer cope with the overload.
The health of patients with mitral stenosis is frequently affected by complications, which may produce new disabilities or aggravate existing ones:
Atrial fibrillation . Elevated pressure in the left atrium resulting in its dilatation is a powerful contributing factor to atrial fibrillation, present in the majority of patients older than 50 years of age. The onset of atrial fibrillation, as a rule associated with a fast heart rate, is likely to produce disabling dyspnea or heart failure. However, response to treatment is usually satisfactory: as soon as the heart rate is slowed, the patient's symptoms abate or disappear.
Left atrial thrombi with emboli . Atrial fibrillation in the presence of a dilated left atrium facilitates the formation of thrombi attached to the atrial wall. Some portions of the clot may break loose and travel in the bloodstream as emboli, the most serious consequence of which is stroke—a relatively common complication of mitral stenosis. Embolic strokes are often reversible; that is, muscle paralysis and speech disturbance may only be temporary. Some patients, however, may suffer permanently disabling or even fatal strokes in the course of mitral stenosis.
Respiratory infection . Mitral stenosis affects blood flow through the lungs. The resulting congestion facilitates upper respiratory infections. Mild infections are inconsequential, but more-severe infections may greatly increase disability and precipitate heart failure.
Pulmonary hemorrhage . Spitting up of blood occurs commonly in mitral stenosis and is of no significance. Serious hemorrhaging from the lungs, requiring blood transfusion, is rare.
Pulmonary edema . Some patients with mitral stenosis are prone to pulmonary edema, particularly after eating salty food. Though potentially dangerous, pulmonary edema is easily treated, and recurrences can be prevented by restricting dietary salt or administering diuretics.
Severe pulmonary hypertension . In most cases of mitral stenosis elevation of pulmonary arterial pressure is only moderate. But in some patients small pulmonary vessels may react abnormally to
elevated pressure in the left atrium by developing severe pulmonary hypertension. This condition seriously compounds disability and may cause intractable heart failure.
Mitral stenosis can generally be diagnosed by performing a physical examination, though evaluation of its severity requires further tests. Among noninvasive tests, electrocardiographic changes and the size and shape of the cardiac shadow in a chest X ray are usually the first indicators in the diagnostic evaluation; an echocardiogram provides further data for estimating the severity of the stenosis and its effect on the circulation. The size of the stenotic mitral orifice can be calculated by means of cardiac catheterization, usually combined with angiography. These two invasive tests are typically performed when surgical treatment is being considered, in which case the extent of pulmonary hypertension and the presence or absence of mitral regurgitation and, in older patients, coronary-artery disease need to be determined.
Surgical repair or replacement of a stenotic mitral valve can not only restore adequate blood flow but also reverse all the secondary effects of mitral stenosis, even in the late stages of the disease. However, several cautionary factors must be considered before deciding on surgery. First, none of the available interventions is capable of restoring fully normal valvular function. Second, early disability in mitral stenosis may be amenable to effective nonsurgical treatment. Third, mitral stenosis often remains stable for years or even permanently, and moderate disability can be controlled by adjustments in life-style. Thus the consensus is that prime candidates for surgery are patients in congestive heart failure or otherwise seriously disabled who are unresponsive to intensive medical treatment.
Several kinds of medical (nonsurgical) treatment are available to patients with mitral stenosis. Diuretics, reinforced by restrictions on salt in the diet, can control fluid retention and the early stages of heart failure. Atrial fibrillation can be treated either by reducing the excessive ventricular rate or by restoring sinus rhythm. To inhibit clot formation, anticoagulants may be administered to certain patients, particularly those experiencing atrial fibrillation. Modification of physical activities may also be recommended.
Surgical treatment of mitral stenosis includes various approaches. In closed mitral valvotomy (without the use of the heart-lung machine)
the surgeon introduces a finger (or sometimes a special instrument) into the left atrium of a beating heart to break the adhesions between the two leaflets. This method, the first used in treating mitral stenosis, was the only one available until pump-oxygenators were developed. Open mitral valvotomy (with the use of the heart-lung machine) permits other techniques for separating the leaflets under direct vision. Mitral valvotomy has the advantage of preserving the natal mitral valve and is particularly successful in younger patients in whom the leaflets have not yet stiffened or calcified. A third alternative is replacement of the natal valve with a prosthetic valve or transplanted biological valve. A recent development, invasive but nonsurgical dilatation of the mitral valve (percutaneous balloon mitral valvuloplasty), has been shown to produce satisfactory results in some patients. The long-term effects of this procedure are not yet known.
The dramatic decrease in the incidence of rheumatic fever since the 1940s has greatly reduced the number of cases of mitral stenosis in the Western countries. Most persons in the United States treated for mitral stenosis are over the age of 50; many return for treatment because of the recurrence of stenosis following mitral valvotomy in the distant past or because of malfunction of prosthetic valves. Younger patients with mitral stenosis are mainly immigrants from less-developed countries.
Aortic Stenosis
Stenosis of the aortic orifice is now the commonest valvular disease; it is seen predominantly among the elderly. The term "valvular aortic stenosis" denotes stenosis caused by a stiffened valve and is distinguished from narrowing below or above the aortic valve, occasionally seen as a variety of congenital heart disease.
There are two principal ways in which the aortic valve may become stenotic—fusion of the three valve leaflets, due to adhesion among them, or stiffening and calcification of the leaflets, which may keep the valve from fully opening, leaving a greatly reduced orifice during systole (see fig. 32, p. 130). In addition, aortic stenosis may be present since birth, as a congenital defect in which the three leaflets have grown together, leaving open a small central orifice that can still function normally during diastole.
Acquired fusion of the leaflets by adhesions among them is almost always the result of rheumatic fever and, as in mitral stenosis, takes decades to develop. Rheumatic aortic stenosis, however, is now relatively uncommon. Today most cases are considered a type of "calcific aortic stenosis," a gradual fibrotic thickening of the leaflets in which the deposition of calcium can eventually make the valve leaflets as hard as bone. Normal leaflets, paper-thin, open wide in systole, permitting easy flow of blood through the aortic orifice. The slightest deviation from the norm may alter the flow patterns through the valve and in time cause minor damage to the valve. This damage may then produce fibrosis and eventually the deposition of calcium. A common cause of calcific aortic stenosis is a minor congenital malformation, namely an aortic valve consisting of two rather than three leaflets. Such a valve functions normally, and its presence is seldom recognized. But any minor change in the shape of the orifice may initiate the process that can produce aortic stenosis after a period of sixty to seventy years.
Thus aortic stenosis is a disease of old age, with most patients in their sixties, seventies, or eighties. This process progresses slowly, so that many years usually elapse from the time aortic stenosis is first detected until it becomes severe enough to cause symptoms. The normal aortic orifice during systole measures 3–5 cm.2 When it reaches about one-half of its normal size, turbulent flow produces a heart murmur. However, the overload on the left ventricle does not develop until the orifice is reduced to less than 1.5 cm.2 In moderate aortic stenosis the orifice has narrowed to 0.7–1.0 cm2 ; in severe aortic stenosis the orifice may be as small as 0.4 cm.2 The degree of hypertrophy of the left ventricle depends on the severity of stenosis; hence the first manifestation of left ventricular failure may become apparent only once the stenosis is severe. The patient then is likely to become aware of shortness of breath during exercise. Before the onset of left ventricular failure, however, some patients develop symptoms unique to aortic stenosis, related to the extreme pressure in the left ventricle and the slow ejection of blood through the stenotic aortic orifice. Certain reflexes originating in the left ventricle may interfere with the regulation of blood pressure and produce sudden loss of consciousness (syncope); furthermore, blood flow through the coronary arteries may be affected by aortic stenosis, producing anginalike chest pain unrelated to coronary
disease. Children with congenital aortic stenosis usually tolerate it well, although sometimes unusually severe aortic stenosis may bring about emergencies in infancy.
Aortic stenosis can be detected on physical examination by the characteristic heart murmur and a peculiarity of the pulse. The electrocardiogram will disclose left ventricular hypertrophy, which, though a nonspecific finding, usually indicates that aortic stenosis is at least of moderate severity. In older patients a chest X ray may show calcification of the aortic valve. An echocardiogram can display more directly the narrowing of the aortic orifice, the severity of which can be estimated by the Doppler technique. The size of the aortic orifice can be calculated more accurately from data obtained through cardiac catheterization, which reveals the magnitude of the pressure gradient between the left ventricle and the aorta and the volume of blood flow. In older patients cardiac catheterization is usually combined with coronary angiography. Chest pain, if present, may be caused in these patients by aortic stenosis alone or by coexisting coronary-artery disease—an important distinction when deciding on the proper medical or surgical management of the patient.
In contrast to mitral stenosis, where complications play a major role in its course, there are relatively few complications in aortic stenosis. Atrial and ventricular arrhythmias occasionally develop but are relatively rare. Patients with mitral stenosis are susceptible to infective endocarditis, and preventive measures should be taken. Those with advanced aortic stenosis are at higher risk of sudden cardiac death than those with other valvular diseases.
Treatment of aortic stenosis is almost entirely surgical. Relief of the condition represents one of the most spectacular accomplishments of cardiac surgery and is usually effective even in the most advanced cases. There are several approaches, depending on the type of aortic stenosis. Aortic commissurotomy , surgical separation of adherent leaflets through open-heart surgery, is mainly performed on children and adolescents with congenital aortic stenosis. The results are moderately satisfactory, although secondary changes may develop later in life requiring reoperation. Aortic-valve replacement is the standard treatment of aortic stenosis. It is the second most frequently performed cardiac operation (after coronary bypass surgery), and its successes are often brilliant: function of the left
ventricle, if impaired, returns to normal, and hypertrophy of the left ventricle may regress. Percutaneous aortic balloon valvuloplasty has been available only since the mid-1980s; hence its long-range success is uncertain. The advantages of dilating the aortic orifice without surgery are obvious. However, in elderly patients with heavily calcified aortic valves the successful widening of the orifice may not last long, for restenosis is very common. Consequently, this procedure is performed mostly on patients who are poor candidates for open-heart surgery.
The prevailing view is that patients with symptoms clearly related to aortic stenosis should undergo surgery. In the case of an aortic stenosis, even severe, that permits the patient a symptom-free, active life, surgery is usually postponed. Following valve replacement most patients can lead a normal, unrestricted life. Further treatment consists of preventive measures against endocarditis and, in patients with nonbiological valves, anticoagulant therapy. Successful operations are being performed on patients even in their eighties or nineties.
Mitral Regurgitation
The mitral valve is subjected to the highest stresses of the four cardiac valves: it is exposed to the systolic pressure in the left ventricle, at least 120 mm Hg. Its competence is contingent not only on the state and function of its two leaflets but also on the function of the reinforcing auxiliary parts of the mitral-valve apparatus—the chordae tendineae and the papillary muscles of the left ventricle. Consequently, mitral regurgitation may be caused by a variety of mechanisms:
Scarring after rheumatic fever may produce shrinking of the leaflet.
Infective endocarditis may create a hole in a leaflet.
The chordae may stretch, preventing tight closure of the leaflets.
One or more chordae may rupture.
The left ventricular papillary muscle may malfunction and be unable to tighten the chordae.
When the left ventricle dilates, the orifice may become too large for the leaflets to cover it.
The valve may be congenitally deformed.
Mitral regurgitation often develops as a complication of other cardiac diseases. A mild degree of incompetence of this valve has no significant effect on the circulation. Significant mitral regurgitation may also appear as the principal cardiac disease.
Rheumatic mitral regurgitation is most often seen in combination with mitral stenosis, with the latter being the predominant disease. Alone, mitral regurgitation is in some ways similar to mitral stenosis. Although regurgitation in children may be an immediate consequence of rheumatic fever, it is more likely to develop a long time after the attack. Disability tends to appear only in middle age or later. Complications include atrial fibrillation, although embolic episodes are less common than in mitral stenosis. Surgical treatment frequently involves valve replacement, but in some cases the deformed mitral valve can be successfully repaired. In developing countries, where severe recurrent rheumatic fever is prevalent, mitral regurgitation may evolve rapidly, requiring valvular surgery at a young age.
Prolapse of the mitral valve was first identified in the 1960s. Its unique feature is that the volume of blood returning to the left atrium is small and does not increase the workload of the left ventricle. Nevertheless, mitral-valve prolapse has become one of the most widely debated topics in cardiology. The potential complications of this lesion are graver than its direct effect. Mitral-valve prolapse may be associated with the following sequelae:
arrhythmias, both atrial and ventricular
infective endocarditis
embolic stroke (slight but definite risk)
atypical chest pain, which may be mistaken for coronary disease
rupture of some chordae, which can change trivial mitral regurgitation into acute severe mitral regurgitation requiring emergency intervention
In mitral-valve prolapse the leaflets may be larger than normal and stretch the chordae, producing valvular incompetence. Prolapse of the valve means that one leaflet overlaps the other, the most important cause of which is an abnormal structure of the leaflets called mucoid degeneration. This condition is often genetic. Such valves may become progressively larger, with redundant
tissues flapping in the mitral orifice like a parachute. Hereditary degeneration of the mitral valve occurs in young patients, is more common in women than in men, and has a relatively high rate of complications. Yet it accounts for only a small fraction of the cases of prolapse. In general, mitral-valve prolapse is a benign condition most frequently discovered on routine examination and having an excellent prognosis.
A preliminary diagnosis of mitral-valve prolapse may be made simply by listening with the stethoscope (auscultation) for the characteristic heart murmur and an extra sound. The principal test used to establish a firm diagnosis of this disease is the echocardiogram, which shows one leaflet slipping over the other. The echocardiogram may in fact be too sensitive a diagnostic tool: normal mitral valves may have somewhat oversized leaflets, which on the echocardiogram may resemble abnormal prolapse. It is now generally recognized that echocardiographic criteria for diagnosing mitral-valve prolapse have to be critically evaluated to guard against ascribing this valvular disease to healthy young adults.
Patients with mitral-valve prolapse need to be reassured of the favorable prognosis. Some patients require endocarditic prophylaxis. Those with the more pronounced hereditary prolapse are sometimes treated for arrhythmias. In rare cases of severe mitral regurgitation valve replacement may become necessary.
Rupture of the chordae tendineae is one of the causes of acute severe mitral regurgitation. It usually develops as a complication of other cardiac diseases, such as mitral-valve prolapse or infective endocarditis, but may represent a primary mitral-valve disease. Spontaneous rupture of the chords occurs occasionally, especially in older male patients. Chordal rupture may also result from trauma, a nonpenetrating injury to the chest. Mild varieties of chordal rupture usually involve separation of a single chord; it may have a minimal hemodynamic effect and require no treatment. More often, however, rupture involves multiple chords and may create an emergency requiring early valve replacement or repair.
Ischemic mitral regurgitation is a complication of coronary-artery disease, most frequently during or after an acute myocardial infarction. It is caused by damage to one of the two papillary muscles in the left ventricle, which then can no longer pull the chordae
tendineae tight to help make the valve competent. There are two types. (1) Rupture of the papillary muscle or a part of it is a serious, often catastrophic complication of myocardial infarction and usually requires an emergency valvular operation. (2) Malfunction of a papillary muscle may also develop during the course of myocardial infarction but has less serious consequences. Occasionally mitral regurgitation may not be apparent until after recovery from myocardial infarction. The severity of mitral regurgitation due to damage of the papillary muscle varies; furthermore, regurgitation may become progressively more severe. Treatment may be medical or surgical, depending on the severity of the condition and the patient's response to drug therapy. Valve replacement performed because of heart failure produced by mitral regurgitation may be ineffective if serious postinfarction damage to the heart muscle has occurred.
Infective endocarditis may result in new damage to the mitral valve, causing regurgitation in a previously competent valve. This is sometimes the case in patients with mitral stenosis or when the infection develops on a valve with a minor abnormality not affecting its function, such as mitral-valve prolapse. If the infection is seated on an already incompetent valve, the amount of regurgitation may increase, particularly if infection produces perforation of the valve or disrupts the chordae tendineae. Acute mitral regurgitation caused by endocarditis, if severe, may lead to a cardiac emergency requiring immediate valvular surgery.
Rarer varieties of mitral regurgitation include congenital mitral valve clefts (division of one or both leaflets into two parts with a space between them), marked dilatation of the left ventricle in association with heart failure, and cardiac tumors (myxomas of the left atrium), which may interfere with the closure of the valve.
Aortic Regurgitation
There are several causes of incompetence of the aortic valve:
rheumatic damage to the leaflets
perforation of leaflets in infective endocarditis
disease of the aortic root (the initial portion of the aorta just above the valve)
congenital deformity (either combined with other malformations or as a sole lesion)
trauma producing detachment of a leaflet
Two basic mechanisms produce aortic regurgitation—abnormality of the leaflet and dilatation of the root of the aorta (the ring to which the valve is attached becomes widened and the orifice too large for healthy valves to cover it).
Given a comparable volume of blood regurgitating through an incompetent left-sided cardiac valve, aortic regurgitation imposes a greater overload on the left ventricle than does mitral regurgitation. Blood leaking through an incompetent mitral valve into the atrium raises the left ventricular workload mildly; blood returning to the left ventricle through an incompetent aortic valve has to be ejected again into the aorta, steeply increasing the workload. This difference results in a more pronounced hypertrophy of the left ventricle in aortic regurgitation. Furthermore, the large volume of blood handled by the left ventricle dilates it. The dilated, volume-overloaded left ventricle characteristic of aortic regurgitation works less efficiently than the nondilated, pressure-overloaded left ventricle in aortic stenosis.
In the past, chronic aortic regurgitation was caused frequently by rheumatic fever, with or without coexisting mitral stenosis. With the decline in the prevalence of rheumatic heart disease in the West, the commonest cause of chronic aortic regurgitation has become disease of the aortic root, a variety of aortitis affecting the portion of the aorta immediately above the aortic valve (see chap. 14). Syphilitic aortitis was once common, often affecting the aortic valve, but today it is a rarity. Nowadays aortitis may be associated with certain varieties of rheumatoid arthritis or may develop as a result of atherosclerotic disease of the aorta in the elderly.
On physical examination aortic regurgitation displays a characteristic heart murmur. The severity of the regurgitation can be estimated by abnormalities of the arterial pulse and unusually low diastolic blood pressure. Aortic insufficiency cannot be quantified, but a more accurate estimation of regurgitant volume is possible by echocardiography, using the Doppler principle. As a rule, cardiac catheterization contributes little to the diagnosis.
The consequences of aortic regurgitation depend on whether the
lesion develops gradually or abruptly. The increased workload acute aortic regurgitation imposes on a normal left ventricle, if severe, may produce life-threatening heart failure requiring emergency valvular surgery. Chronic aortic regurgitation, by contrast, permits adaptive hypertrophy of the ventricle and is generally well tolerated; patients with severe aortic regurgitation may lead an unrestricted active life for many years. Eventually the left ventricle reaches the limit of its adaptative capacity, leading to cardiac failure. But in aortic regurgitation, more than in other valvular diseases, the onset of the first symptoms (usually dyspnea produced by activity) may develop so late that relief of the overload by valve replacement may no longer restore good left ventricular function. To avert this problem, in aortic regurgitation early deterioration of left ventricular function is occasionally considered an indication for surgery even in asymptomatic patients. When symptoms suggestive of heart failure are present, surgery is mandatory.
The major complication of aortic regurgitation is infective endocarditis. Atrial fibrillation is rare; ventricular arrhythmias may appear during late stages. The prolonged asymptomatic course of aortic regurgitation usually makes medical treatment unnecessary except to prevent endocarditis.
Other Diseases of the Cardiac Valves
Pulmonary stenosis is almost always congenital. It will be discussed in chapter 10. Pulmonary regurgitation is rare. It occasionally develops in severe pulmonary hypertension, without intrinsic disease of the valve leaflets, when high pressure in the pulmonary artery makes the healthy pulmonary valve incompetent. Damage to the pulmonary valve may occur in infective endocarditis, found most frequently in intravenous drug abusers since bacteria introduced with intravenously administered drugs tend to infect right-sided cardiac valves.
Tricuspid stenosis is a rare complication of rheumatic fever. It almost always coexists with mitral-valve disease. Tricuspid regurgitation is a relatively common sequel to pulmonary hypertension. It commonly results in right ventricular failure, when the tricuspid orifice stretches, making the valve incompetent. Its presence merely aggravates the effect of right ventricular failure on the pressure
in the veins bringing blood to the right atrium. Tricuspid-valve repair or replacement is occasionally imperative, but physicians prefer to avoid it because operations on the tricuspid valve are not as effective as those performed on left-sided cardiac valves. Tricuspid-valve involvement may also occur in endocarditis among drug abusers.
Combined diseases of cardiac valves are a relatively common consequence of rheumatic fever involving the mitral and aortic valves. Aortic stenosis or regurgitation resulting from rheumatic fever is more frequent in combination with mitral-valve involvement (usually stenosis) than as the sole consequence of rheumatic fever. The course and prognosis of double valve disease usually follows the pattern of the predominant lesion.
Stenosis of the mitral or aortic valve may be associated with incompetence of the same valve. The consequences of combined stenosis and regurgitation of a left-sided cardiac valve usually differ little from those of pure stenosis; less commonly, however, they follow the pattern of pure regurgitation of the valve.
Infective Endocarditis
The term "infective endocarditis" has replaced the older term "subacute bacterial endocarditis." It is the most serious complication of valvular heart disease, an infection of the cardiac valves caused most often by bacteria and occasionally by fungi but not by viruses. Cardiac valves are not the only sites of infection; congenital heart lesions are also susceptible. It is unusual for endocarditis to develop on healthy valves since microorganisms tend to settle on damaged endocardium.
Bacteria in the bloodstream (bacteremia) is common even in health. A variety of organisms normally present on the skin and in the mouth, nose, and rectum may enter the bloodstream as a result of minor cuts or punctures. The body's defense mechanisms take care of eliminating the bacteria unless they can find a vulnerable spot in the inner lining of the heart and blood vessels; even then only occasionally can they take sufficient hold to cause infection. Certain conditions or procedures are likely to introduce large numbers of microorganisms into the bloodstream and thus carry a higher-than-average risk of starting an infection:
dental surgery
surgical procedures or manipulations involving the gastrointestinal and genitourinary tracts
intravenous injection of drugs among drug abusers
superficial infections such as abscesses
Invasion of microorganisms into a damaged endocardium produces a local reaction. Blood cells accumulate, including platelets and fibrin, which along with small thrombi form a structure, called a vegetation, that attaches itself to the valve. Single vegetations have the appearance of small pearls, but they tend to aggregate, producing larger structures sometimes resembling a bunch of small grapes. These vegetations can damage valvular tissue by producing holes in the valves or actually chewing up portions of them. Moreover, they may detach themselves and float away in the bloodstream, producing emboli. The consequences of infection of valves and the extent of damage are related to the type of microorganism, its aggressiveness (virulence), the extent of blood contamination, and the time antibiotic therapy is started.
The onset of endocarditis may be sudden, characterized by chills and high fever (acute endocarditis). More often, however, endocarditis develops inconspicuously and may not be recognized for weeks or even months (subacute endocarditis). In such cases patients may be aware of a certain lassitude or lack of pep. Fever is almost always present, though it may be slight. Anemia often accompanies subacute endocarditis. The correct diagnosis is frequently apparent, despite the vague symptoms, if the patient is known to be at risk for endocarditis because of valvular or congenital heart disease. However, if the presence of heart disease is undetected—as in the case of trivial valvular lesions—considerable difficulties may arise in interpreting the symptoms.
Endocarditis affects patients in several ways. The infection may spread and cause death. (The mortality rate from endocarditis is about 20 percent.) The destructive process on valves may produce acute, severe valvular regurgitation, leading to heart failure or even shock. Loose vegetations may produce embolic damage to distant organs such as the brain, kidneys, or spleen. Prompt antibiotic therapy can cure the infection without any aftereffects.
Infective endocarditis is suspected in cases of unexplained fever in patients with valvular or congenital heart lesions. A more direct clue to the diagnosis is a heart murmur not present previously. Also of aid in diagnosing the disease is echocardiography, which can detect vegetations provided they have reached significant size. The confirmatory test is a blood culture to identify the organism responsible for the infection. A sample of the patient's blood is placed in a medium, such as broth, on which bacteria thrive and is incubated at body temperature. Once the organism has been identified, the proper antibiotic therapy can be chosen and the prognosis determined.
The commonest cause of subacute infective endocarditis is the green streptococcus (Streptococcus viridans ). But almost every known microorganism capable of invading the body can, under appropriate circumstances, produce endocarditis. Some of them can be cultured and identified within 24 to 48 hours. Others may need special culture mediums and techniques. In 10–20 percent of cases the infecting organism cannot be identified.
Antibiotic therapy is required for all cases of infective endocarditis and is started as soon as the microorganism is identified. If prompt identification from blood culture is not forthcoming, interim therapy with some broadly effective antibiotics should be administered. The effect of therapy is often apparent within a few days, as the fever and malaise disappear; however, antibiotic therapy should be continued for a few weeks after the symptoms have passed, to ensure that all viable infecting organisms have been eliminated from the body. The initial therapy is usually administered intravenously and is later replaced by oral antibiotics. Follow-up blood cultures help in monitoring the success of treatment.
Whether therapy other than antibiotics is indicated depends on the extent of damage to the valves or other infected structures. Endocarditis caused by the green streptococcus is relatively benign and usually requires only antibiotic therapy. Fungi, yeasts, and more-aggressive bacteria may cause extensive valvular damage, often requiring surgical removal of the infected valve and its replacement with a prosthesis. In an emergency such as severe heart failure the surgery may be performed despite the infection, but it is preferable to wait until the organisms are destroyed by antibiotics.
Prosthetic valves themselves can be targets of bacterial infection; infected prostheses usually require replacement.
Preventive therapy should be undertaken in all patients at risk of infective endocarditis. It consists of administering an antibiotic before procedures that may introduce microorganisms into the bloodstream and continuing treatment briefly thereafter. Among conditions requiring antibiotic prophylaxis are dental extractions and surgery, diagnostic and therapeutic procedures in the gastrointestinal and genitourinary tracts, and childbirth. Antibiotic prophylaxis does not offer total protection but does reduce the probability of infecting the heart.