Chapter Eight
Atherosclerosis and
Coronary-Artery Disease
Atherosclerosis is a specific disease of the intima (inner layer) of the arteries, not necessarily related to aging, and is responsible for the most prevalent serious disease of the heart, coronary-artery disease. It typically affects the aorta and the arteries supplying blood to the heart, the brain, and the lower extremities. It should not be confused with arteriosclerosis , commonly known as hardening of the arteries, a degenerative process affecting the arteries that is a part of aging. It affects the arterial intima as well as the media (middle layer), producing abnormalities that generally do not have adverse effects on the flow of blood in the arteries.
Atherosclerotic Coronary-Artery Disease
Atherosclerosis does not uniformly affect the arterial intima but is localized, involving small foci inside the arteries. It is only when the localized lesions become large enough to interfere with the flow of blood that atherosclerosis produces serious consequences. Coronary arteries are the most vulnerable to lesions restricting blood flow because the heart has the highest rate of using and extracting oxygen from the blood and is very sensitive to its lack. Furthermore, coronary arteries do not have significant interconnections between their branches to offer alternate routes of blood supply when obstruction develops. Atherosclerosis causes a variety of lesions inside coronary arteries; from the standpoint of heart disease,
however, the most important lesion is the atherosclerotic plaque , a small blisterlike protrusion of the intima into the lumen (cavity) of the artery, which can produce stenosis.
The atherosclerotic plaque consists of firm fibrous tissue surrounding a softer center containing fatty substances, cholesterol crystals, and other debris. A plaque in a coronary artery does not significantly interfere with blood flow until the obstruction reaches at least 60 percent of the lumen. The development of a plaque of sufficient size to interfere with blood flow is a slow process, usually taking many years. When the plaque begins to affect blood flow, large collateral channels connecting the branches of the affected artery with those of other coronary arteries may start to develop, thereby supplying the needed blood. The normal interconnections between healthy coronary arteries are tiny branches capable of supplying only minute quantities of blood to the occluded artery in an emergency. However, the gradual progression of coronary stenosis stimulates growth of these collateral interconnections, which often become large enough to take over the entire blood supply from a completely occluded artery. Thus nature may provide a bypass to a stenosed or occluded coronary artery.
The most serious consequences of coronary atherosclerosis are produced by certain complications of this process. These are
rupture of a plaque
hemorrhage into the wall of an artery
formation of a thrombus inside an artery
Each of these complications increases abruptly the degree of stenosis, which usually results in a significant progression of symptoms and other consequences of coronary disease.
In rupture of a plaque (referred to as a "coronary accident"), much as in rupture of an abscess, the fibrous covering splits, permitting its soft contents to spill into the artery. In response, white blood cells and platelets immediately aggregate at the point of rupture to repair the damage. This process not only increases the stenosis but may also stimulate the formation of a thrombus, which could further reduce the lumen of the affected artery or close it off completely. The effect of this kind of accident on the heart depends on how much increase in stenosis has taken place and on whether
compensatory collateral arteries are available. Hemorrhage into the arterial wall (subintimal hemorrhage ) can also suddenly increase the degree of stenosis, although its effects are seldom as dramatic as those of rupture of a plaque. Thrombus formation inside a coronary artery, though a common sequel to plaque rupture, may also occur without it, stimulated by an area of damage in the intima. Coronary thrombosis is the principal cause of myocardial infarction.
The cause of atherosclerosis is not definitely known. Among theories dealing with the initiation of the atherosclerotic process the most widely accepted is the injury hypothesis, which suggests that a localized injury to the intima of a coronary artery is the point of origin of atherosclerotic lesions. It is well recognized that the early stages of atherosclerosis may develop even in adolescents and children. Yellow fatty streaks, the visible precursors of atherosclerotic plaques, are frequently found in arteries of healthy young adults. Local injury to the intima may attract fatty substances, which are then deposited there, initiating a rather complex process leading eventually to large, clinically significant plaques.
Atherosclerotic coronary-artery disease is not distributed evenly around the globe. Its incidence in the developing nations is much lower than in developed countries; in some tribes of Africa it is virtually nonexistent. A major reason for this difference is the amount and type of fatty substances in the diet. Diets rich in foods containing saturated fatty acids and cholesterol, such as prevail in most Western nations, enhance the development of coronary disease.
The principal building block of atherosclerotic plaques is cholesterol—a ubiquitous substance, important in all cell structures, which in combination with proteins is transported in the bloodstream as lipoprotein . Among the various types of lipoproteins two play important roles in the development of coronary atherosclerosis: low-density lipoproteins (LDLs), which transport most of the cholesterol and tend to deposit it in arterial lesions, and high-density lipoproteins (HDLs), which are capable of removing cholesterol from injured arterial walls and transporting it to the liver for use in forming bile.
The relationship between the amount of cholesterol circulating in the blood (serum cholesterol) and coronary-artery disease is well
documented, and the level of serum cholesterol is used as a predictor of coronary disease. That level in the Western population ranges between 150 and 300 mg per 100 milliliters of serum (mg percent). Serum cholesterol is greatly influenced by the amount of saturated fat and cholesterol in the ingested food, although the varying ability of persons to metabolize fatty substances plays an important role as well. The present view is that a desirable level of serum cholesterol in adults is less than 200 mg percent, and a level above 240 mg percent is too high and may require intervention to reduce it. Whereas total serum cholesterol is the widely used risk factor, it is now generally accepted that LDLs ("bad cholesterol") are implicated in atherosclerotic plaque formation, whereas the HDLs ("good cholesterol") inhibit plaque formation. Higher ranges of serum cholesterol may reflect a high-fat diet or inefficient fat metabolism. In one disease, familial hyperlipidemia (it is usually hereditary), grossly abnormal metabolism of fatty substances may raise serum cholesterol as high as 1000 mg percent. There are several varieties of this metabolic disorder, most of which are associated with premature and severe coronary-artery disease.
Triglycerides , another group of chemical substances transported with lipoproteins, are also considered predictors of coronary-artery disease if serum levels are abnormally high, although this relationship is not as clear as that involving LDL cholesterol.
The atherosclerotic process affects men more often than it affects women. It is thought to be stimulated or retarded by the presence or absence of certain risk factors. Inasmuch as the relationship between risk factors and coronary-artery disease is based on statistics rather than direct observation, the importance of these risk factors is not always clear-cut and is the subject of controversy. Risk factors that can be influenced by therapy are of particular importance since their reduction may arrest the progress of atherosclerosis or even cause its regression. Others merely identify subjects at higher-than-average risk of heart attacks.
The three risk factors accepted by most experts as central to address in preventive treatment of atherosclerosis are hypercholesterolemia (high serum cholesterol), smoking, and hypertension (high blood pressure). Risk factors whose role is less established or is questionable include stress, obesity, sedentary habits, diabetes, and the so-called coronary-prone personality. Risk factors that cannot
be altered by treatment include male sex and a family history of heart attacks early in life.
Risk factors for coronary-artery disease should be addressed whenever possible by prophylactic treatment. The most aggressive and widely accepted treatment is aimed at reducing serum cholesterol. It includes two strategies—primary prevention, directed at the entire population of a given area, and secondary prevention, directed at patients who have had heart attacks or have shown other manifestations of the disease.
Primary prevention involves modifying one's diet. Since dietary restrictions may affect quality of life, primary prevention of coronary-artery disease focuses on the least disruptive adjustments in dietary habits. A healthy diet for children and adolescents, if followed by a large segment of that population, could have a significant impact on the future prevalence of coronary-artery disease. Stronger measures, such as drug therapy, are generally indicated only in persons with significant hypercholesterolemia.
Secondary prevention requires a more aggressive approach, including a stricter diet and, if indicated, drug therapy. Two classes of drugs reduce serum cholesterol—those affecting fat metabolism in the body and those interfering with fat absorption in the bowel. The latter drugs may cause gastrointestinal upset but are otherwise well tolerated and are considered safe for long-term, often lifelong, use. Powerful drugs affecting fat metabolism have now been accepted for general use, but not enough time has elapsed to determine whether long-term use of these effective drugs carries any risk to vital organs. They therefore are administered mainly when dietary means fail and the level of serum cholesterol is unusually high.
Complementing these preventive treatments of coronary-artery disease are agents that reduce the risk of thrombus formation. Evidence suggesting that small doses of aspirin may inhibit blood platelets from contributing to thrombus formation has provided a simple and inexpensive means of influencing the process that leads to heart attacks. However, the practical value of aspirin in preventing heart attacks has yet to be clearly established.
Risk modification concerning high blood pressure and smoking is treated as a general health measure rather than as a specific preventive measure in coronary-artery disease. Hypertension requires
treatment irrespective of its role in coronary-artery disease; similarly, the noxious effect of tobacco, the subject of intensive antismoking campaigns, involves other diseases than atherosclerosis. The question whether risk modification can actually produce regression of existing atherosclerotic lesions has not yet been answered, though some studies suggest it can.
Sequelae of Coronary Atherosclerosis
Coronary-artery disease is related to a single consequence of atherosclerotic reduction of coronary blood flow—ischemia of the heart muscle. Myocardial ischemia is a decreased delivery of oxygen to heart muscle cells such that myocardial oxygen demand exceeds the supply of oxygen through the coronary circulation. Thus ischemia may develop if myocardial oxygen requirements increase but supply remains the same or if such requirements remain stable but blood flow supplying oxygen is reduced. Increased myocardial oxygen demand is related to cardiac workload. For example, exercise steeply increases the oxygen requirements of the heart muscle; a similar effect, though less pronounced, is produced by factors elevating arterial pressure.
To explain the relationship between oxygen supply and demand, we need to understand the concept of coronary reserve . Approximately 20 percent of the maximum capacity of the coronary arteries to supply the heart muscle with oxygen is required by a person at rest. The remaining 80 percent of potential blood flow represents the coronary reserve available to take care of increased cardiac oxygen demands. An atherosclerotic plaque may grow to the point where coronary blood flow is affected, reducing the coronary reserve from 80 percent to 60 percent. In this case myocardial ischemia would develop only when the full reserve was required, such as during strenuous exercise, at which time the person would experience chest pain. Further growth of a plaque eliminates more cardiac reserve, producing chest pain with less-strenuous activity. More-severe stenosis may reduce coronary reserve to the point where relatively light activity produces ischemia. Myocardial ischemia (and its accompanying chest pain) may also result from other factors raising myocardial oxygen demands, such as increases in blood pressure or heart rate due to stress or excitement. Ischemia
caused by increased myocardial oxygen demands is by definition reversible, for adequate oxygen supply resumes as soon as oxygen requirements return to normal (basal) levels.
Myocardial ischemia caused by reduced blood supply without any increase in myocardial oxygen demand may be temporary. A spasm of a coronary artery may restrict normal blood flow through it, and normal myocardial function returns once the spasm is relieved. More serious is a permanent injury to the myocardium, which may develop if ischemia persists for at least 15 to 20 minutes (according to present estimates). The ultimate variety of reduced blood and oxygen supply to the myocardium is permanent occlusion of a coronary artery by a thrombus, resulting in myocardial infarction.
Myocardial ischemia can have several effects on the heart—faulty contraction of the affected portion of the heart muscle (reduced or absent contractions); left ventricular failure, if the ischemia affects a large area of the myocardium; and, occasionally, ventricular arrhythmias. The consequences of temporary ischemia disappear either spontaneously or in response to interventions (such as taking nitro-glycerin), provided adequate blood supply is restored within the critical time limit.
Ischemia can be detected by means of the following diagnostic findings:
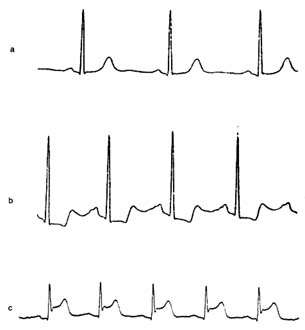
electrocardiographic changes consisting of a temporary shift of the S-T segment (fig. 27)
reversible abnormalities of isotopic perfusion scans performed in connection with stress tests (fig. 16, p. 39)
reversible abnormalities of left ventricular wall motion
Electrocardiographic abnormalities characteristic of ischemia may show up over the course of a Holter monitor test or during a treadmill exercise test. They may also appear when monitoring hospitalized patients during a spontaneous attack of chest pain.
In the treadmill stress test the workload on the heart is gradually increased by speeding up the treadmill and adding a slight incline. The goal of the test is to attain 90 percent of the predicted maximal heart rate during exercise for the person being tested (ranging between 120 and 150 beats a minute). A multilead electrocardiogram records during the test; the patient is also monitored for
Figure 27. Single electrocardiographic leads exemplifying changes
produced by myocardial ischemia. (a) Normal configuration.
(b) Complexes showing depressed S-T segments, signifying moderate
myocardial ischemia (e.g. during treadmill exercise test), which return
to normal when the normal flow is restored. (c) Complexes showing
elevated S-T segments signifying severe ischemia (myocardial
injury), such as seen during early stages of myocardial infarction.
changes in blood pressure and the development of chest pain. In the presence of ischemia the S-T segment of the electrocardiogram shifts downward or, if ischemia is unusually severe, upward. Information concerning the severity of ischemia can be obtained from the extent of the S-T segment shifts, the amount of exercise required to provoke ischemia, and the time needed for the S-T segment to resume a normal position after the test. —
A Holter monitor test may reveal ischemia in the development of S-T segment depression during daily activities. Special equipment sensitive to S-T segment changes must be employed (the
primary objective of the Holter monitor test is the detection of arrhythmias). Spontaneous attacks of angina in hospitalized patients provide an additional opportunity to detect ischemia (or support its diagnosis) by observing temporary S-T segment shifts in the electrocardiogram during the attack of chest pain.
The diagnosis of ischemia by means of electrocardiographic abnormalities does have limitations. This method is most reliable when the control electrocardiogram (taken when the patient is at rest) is normal. Certain abnormalities in the control electrocardiogram may reduce the reliability of a test or even make a conclusive diagnosis of ischemia impossible.
The diagnostic use of isotopic scanning tests has been described in chapter 3. The test is performed in conjunction with the treadmill or pharmacological stress test and complements its findings. Whereas some indication of the severity of ischemia can be obtained from electrocardiographic changes, isotopic scanning can reveal the amount of heart muscle affected by the ischemia. Isotopic scan is of particular importance in cases where the electrocardiographic test is inconclusive or is incapable of supplying diagnostic information because of preexisting distortion of the complexes.
Under ordinary circumstances the electrocardiographic treadmill stress test is the principal means of diagnosing ischemia. Other procedures, such as the isotopic perfusion test and nuclide ventriculogram, steeply increase the cost of the diagnosis and are carried out only if specifically indicated.
Once ischemia has been diagnosed, further evaluation of the extent of coronary-artery disease is often made by means of coronary angiography (see chap. 3). The film taken after injecting the contrast material into the coronary arteries shows the distribution of blood through each coronary-artery system and displays any stenosis and occlusion of the branches (fig. 28). Angiography can also reveal collateral connections between arterial branches. This test is essential for determining the precise location of atherosclerotic plaques and evaluating the extent of coronary-artery disease. It is a prerequisite to such interventions as coronary angioplasty or bypass surgery. It should be emphasized, however, that tests for ischemia and angiography complement each other: angiography shows the lesion that may be responsible for ischemia but does not prove the presence of ischemia.
Figure 28. Coronary arteriograms (in actuality only one artery is visualized at a time).
(a) Normal coronary arteriogram. (b) Coronary arteriogram showing four areas of
severe stenosis (indicated by shaded circles). (Reprinted, by permission, from
Arthur Selzer, Principles and Practice of Clinical Cardiology
[Philadelphia: W. B. Saunders, 1983]).
Features of Coronary-Artery Disease and
Its Course
Myocardial ischemia is the common denominator for all manifestations of coronary-artery disease. Thus a disease responsible for more deaths than any other condition is the result of a single mechanism caused by lesions obstructing the coronary arteries. Interestingly, there is no general agreement on one term to describe the effect of ischemia on the heart. The logical and most appropriate term to incorporate all consequences of ischemia, "ischemic heart disease," has not been widely accepted and is only occasionally used. Less precise terms such as "arteriosclerotic heart disease," "coronary-artery disease," "coronary heart disease," or simply "coronary disease" are predominantly used in medical writings and parlance. Its course and outcome depend entirely on how large a portion of the heart muscle is deprived of oxygen and for how long. Coronary-artery disease occurs in the form of specific syndromes categorized according to their features, prognosis, and need for intervention. In a sense they represent stages of the disease, though the patient does not necessarily progress steadily from milder to more serious syndromes.
Manifestations of coronary-artery disease fall into two categories—(1) acute short-term illness or (2) a chronic state with continuous
or intermittent symptoms. Chronic coronary syndromes include
asymptomatic coronary disease (including silent ischemia)
stable angina pectoris
chronic pump failure
The acute coronary syndromes are
sudden coronary death
acute myocardial infarction
unstable angina pectoris
Since pump failure is the final stage of coronary-artery disease and usually follows myocardial infarction, it will be discussed last in this chapter.
Asymptomatic Coronary Disease
Atherosclerotic plaques have to reach considerable size before producing myocardial ischemia, which usually causes patients to experience chest pain (angina pectoris). Because of the prevalence of coronary-artery disease in the West and the slow growth of most atherosclerotic plaques, some coronary-artery disease can be found in a large segment of the population, as shown by autopsies of healthy persons who died a violent death (such as were performed on the bodies of young soldiers killed in the Korean War). Thus symptom-producing coronary-artery disease represents only a fraction of the incidence of such disease in the general population.
In asymptomatic coronary-artery disease atherosclerotic plaques have not reached the critical size to interfere with coronary blood flow. However, in a subgroup of persons with asymptomatic coronary-artery disease, ischemia is induced much as in stable angina pectoris, but no chest pain results. This phenomenon, commonly known as silent ischemia, can be detected by conventional diagnostic methods, such as the treadmill exercise test, isotopic perfusion test, and Holter monitor test. The reason why myocardial ischemia in some instances fails to be signaled by chest pain has not been adequately determined. Many patients with stable
angina pectoris suffer from attacks of silent ischemia in addition to attacks of chest pain; that is, myocardial ischemia occurs more frequently than they are aware of.
The discovery of silent ischemia in otherwise healthy persons, usually during a routine checkup, presents a dilemma. It is not yet known whether the prognosis of such patients is worse than that of similar people who do not show ischemia. It is uncertain whether the performance of invasive (angiographic) studies in cases of silent ischemia is indicated and whether intervention other than primary prevention of atherosclerosis is advisable.
Stable Angina Pectoris
Angina pectoris is the classical earmark of coronary-artery disease. Stable angina pectoris, a chronic state, can persist unchanged for years. It indicates that a patient with reduced coronary reserve has no symptoms at rest, but during exercise or activities the amount of oxygen supplied to the heart by the coronary circulation may become insufficient to cover the increased demands. The threshold for provoking chest pain may be constant (that is, the same amount of exercise may always produce pain), but often the appearance of chest pain is contingent on an additional factor, such as walking in cold weather or having eaten a heavy meal. Some patients develop angina in response to excitement or anger. Since in stable angina pectoris a provoking factor is responsible for chest pain, a careful analysis of the circumstances leading up to the appearance of pain is vital. For example, an attack of chest pain at night may prove to have followed a nightmare and thus have been provoked. By contrast, unprovoked nocturnal pain is a sign of unstable angina.
Chest pain in stable angina subsides with rest or disappears promptly when the patient takes sublingually a tablet of nitroglycerin. The effect of stable angina on the life-style of patients varies greatly. Mild angina may be controlled without medication by eliminating strenuous exercise. When anginal attacks begin to interfere with ordinary activities, medical treatment may be able to alleviate the symptoms. Gradual changes in stable angina occur in both directions: chest pain may develop during less-strenuous activities than in the past or may only be provoked by more-strenuous exercise than previously. Occasionally angina may disappear altogether.
An apparent worsening of symptoms is best explained by slow progression of atherosclerotic disease, and seeming improvement by development of effective coronary collaterals. Only when acceleration of angina occurs abruptly should the diagnosis of unstable angina be made.
Prognosis of stable angina pectoris is in principle favorable. Statistical studies of large numbers of patients have shown that those with stable angina who have not sustained ischemic damage to the heart muscle (caused by myocardial infarction or a lesser coronary syndrome) have a life expectancy only slightly lower than that of other their age in the general population. In some patients the benign course is interrupted by one of the more dangerous acute syndromes, but they are obviously in the minority. For purposes of prognosis it is customary to subdivide patients with stable angina into those whose coronary angiogram shows significant disease in only one coronary artery, those with "two-vessel disease," and those with "three-vessel disease." However, statistical differences in life expectancy among these three groups is rather small so long as the left ventricle remains healthy.
Evaluating stable angina pectoris requires a general survey of the cardiac status. The possibility that noncoronary ischemia (such as results from aortic stenosis or hypertrophic cardiomyopathy) may be responsible for the symptoms has to be considered. The performance of the heart must be reviewed to determine whether it has suffered myocardial damage. After the presence of ischemia has been confirmed by a treadmill stress test or other diagnostic procedures, many physicians arrange for coronary angiography, though others omit invasive tests if the patient promptly and satisfactorily responds to medical treatment.
Treatment of stable angina involves one of two approaches—medical therapy or interventional therapy (including coronary angioplasty and coronary bypass surgery). Medical therapy of stable angina pectoris is based primarily on the ability of nitroglycerin to provide rapid relief from chest pain. This drug is effective in the form of small tablets that dissolve when placed by the patient under the tongue, once the sole method to control anginal attacks. The 1970s and 1980s saw the introduction of some other effective drugs for preventing attacks of angina. Their use has revolutionized medical therapy of angina pectoris. They are beta-adrenergic blocking
agents, calcium channel blocking agents, and long-acting nitrates having nitroglycerinlike effect. In addition, nitroglycerin can now be administered in slow-release form, as tablets or ointment in patches placed on the skin.
Interventional therapy is effective in controlling angina and is used widely. There is no consensus, however, regarding its indications. The most widely accepted, conservative criteria for intervention include the following:
stenosis of the left main coronary artery
failure to respond to medical therapy
the presence of coronary lesions shown by angiography to be precarious (such as proximal stenosis of the left anterior coronary branch)
evidence that ischemia involves large sections of cardiac muscle
Coronary angioplasty was developed in 1978 and has been a popular alternative to bypass surgery (see fig. 19, p. 55). The technique of coronary angioplasty is described in chapter 4. Originally conceived as a method of dilating proximal stenosis of one of the three major coronary branches in single-vessel disease, the use of this procedure has now been extended to handling lesions in smaller coronary branches and in two- or three-vessel disease.
Angioplasty has proven immensely successful in eliminating or reducing stenosis of coronary arteries by compressing atherosclerotic plaques. However, in about 5 percent of cases the procedure can damage the arterial wall and close off the affected artery altogether. In such cases an immediate bypass operation may have to be performed: standby surgical facilities should be available in hospitals routinely performing angioplasty. Another possible complication of angioplasty is recurrence of stenosis within weeks after the procedure, which develops in 20–30 percent of cases. Repeat angioplasty is usually performed, and the probability of recurrent restenosis is greatly reduced. The advantages of angioplasty over bypass surgery are obvious: it is the simpler of the two procedures, its cost is lower, and recovery is swift.
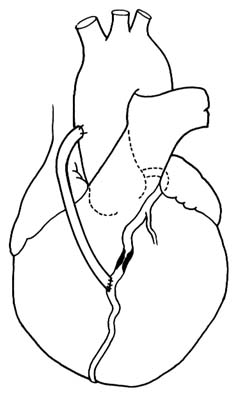
Coronary bypass surgery provides an artificial channel connecting the aorta with the coronary artery beyond the area of stenosis or occlusion (see figs. 29 and 30). The operation is performed under
Figure 29. Coronary bypass graft. A portion of a saphenous vein
from the patient's leg is used to connect the ascending
aorta with a coronary branch beyond the point of severe stenosis.
direct vision as open-heart surgery: a segment of a saphenous vein (a superficial vein running under the skin of the inner surface of a leg) is excised and then grafted to serve as a connecting tube between the aorta and the coronary artery. An alternate method is to connect the lower end of the mammary artery (an artery running inside the chest on either side of the breastbone) with the obstructed coronary artery.
Coronary-bypass surgery is now the most frequently performed cardiovascular operation. More than three-hundred thousand of these procedures are performed in the United States annually. The risk of the operation is relatively small, with mortality rates of 1–5 percent in hospitals with experienced cardiac surgical terms. Patients
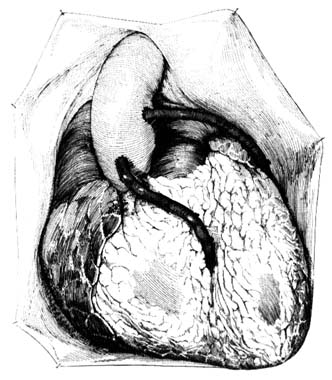
Figure 30. Coronary bypass graft as seen with the chest open.
generally tolerate the operation well, and results are usually satisfactory, although closure of the bypass graft occurs in a significant number of cases, particularly if the graft is connected to a smaller coronary branch.
Interventional treatment of stable angina as well as coronary-artery disease does not represent a cure. Apart from unsuccessful results of the intervention (restenosis after angioplasty, graft closure after an operation) progression of the disease in other coronary branches may bring about new symptoms or more-serious manifestations of coronary-artery disease. Repeat operations or angioplasties are often needed even if the original procedure is fully successful. Yet interventional treatment may delay the progression of the disease by years or even decades. Furthermore, enhanced quality of life and freedom from symptoms as a result of interventional
therapy have made it an exciting advance in the management of heart disease. However, statistical proof that patients who have undergone interventional treatment live longer than those treated medically is not yet available, except in some special high-risk situations. This lack of proof is in part related to the fact that the prognosis of patients with stable angina pectoris who have never suffered a myocardial infarction and have normal function in the left ventricle is good.
Unstable Angina Pectoris
Inherent in the concept of stable angina pectoris is the principle that myocardial ischemia is caused by increased cardiac demand for oxygen. Attacks of chest pain are predictable since each attack has a definable cause, such as exercise or excitement. This predictability is missing in attacks of unstable angina, where oxygen supply to the heart muscle fluctuates irrespective of myocardial oxygen demands.
Unstable angina pectoris, in the broadest sense of the term, includes many situations in which chest pain does not follow a chronic, repetitive pattern. It occupies an intermediate position between stable angina and myocardial infarction and is classified as an acute coronary syndrome. As such, we can distinguish the following patterns:
rapid increase in frequency and severity of attacks of angina pectoris ("crescendo angina")
onset of angina provoked by a low level of activity
a combination of exercise-induced attacks of angina and unprovoked attacks at rest
occasional recurrent attacks of angina at night
a high concentration of unprovoked anginal attacks at rest (usually several attacks a day)
prolonged attacks of angina at rest (15 minutes or longer)
Current medical opinion places the cause of unstable angina close to that of myocardial infarction, namely an acute change inside a major coronary artery, most commonly rupture of an atherosclerotic plaque. Though the course of unstable angina is unpredictable,
the six patterns are listed here in order from least to most precarious. All cases of unstable angina should be considered for intensive hospital treatment, which is mandatory in the more serious varieties. Prolonged attacks of chest pain at rest are usually handled as suspected myocardial infarctions. Since the difference between unstable angina and myocardial infarction depends on the reversibility of ischemia, sometimes the two can be distinguished only after noting a series of changes in electrocardiographic readings and cardiac enzymes. Rapidly recurring attacks of angina at rest that are of shorter duration often are precursors of myocardial infarction. In some patients suffering occasional recurrent attacks of nocturnal angina, unstable angina may be caused by periodic spasm of a coronary artery (analogous to attacks of migraine caused by spasm of cerebral arteries). Coronary-artery spasms have distinctive features. An electrocardiogram taken during such an attack shows an elevated S-T segment instead of the depressed one typical of ischemia at rest. This angina, called variant angina or Prinzmetal's angina, displays electrocardiographic patterns identical with the early changes of myocardial infarction, but the patterns return to normal promptly after the attack. Many such patients have only a minor degree of coronary-artery disease; sometimes in fact the coronary arteries are entirely normal. The prognosis for such patients is much more favorable than for those with other varieties of unstable angina, and their response to medical treatment is usually excellent; in these cases intervention therapy does not help and is contraindicated.
Management of unstable angina is aimed at prompt control of symptoms for patients both in the hospital and at home. Hospitalized patients usually require continuous intravenous administration of drugs. In addition to antianginal drugs, anticoagulants (to prevent clot formation) and thrombolytics (to dissolve clots) may be used. Interventional therapy is often considered, requiring coronary angiograms. Both angiography and interventional treatment may have to be performed as emergency procedures if symptoms cannot be controlled by medical therapy.
As an acute coronary syndrome, unstable angina has an unpredictable outcome: it may progress to myocardial infarction, it may settle into stable angina, or it may cease altogether or change into stable angina. If interventional therapy is performed, either angioplasty
or bypass operation may restore the patient to an asymptomatic state.
Acute Myocardial Infarction
Myocardial infarction—a heart attack, in common parlance—affects at least three-quarters of a million Americans a year. It is an acute event in the course of atherosclerotic coronary-artery disease; after the initial attack of chest pain it takes approximately six weeks for the body to repair the damage to the heart muscle by forming a firm scar. Present standards of care require that the early treatment be administered in the intensive-care unit of a hospital (or in a coronary-care unit if available), followed by further care at a routine hospital facility. The average hospital stay is one week, with wide variations depending on the type of myocardial infarction and the possibility of complications.
The term "infarction" means damage to a tissue in the body caused by depriving it of blood supply. Myocardial infarction results from myocardial ischemia that has lasted too long for the affected tissue to recover and has become irreversible, thereby producing necrosis (death associated with softening) of a portion of the heart muscle. Myocardial infarction is almost always caused by an intracoronary accident (described earlier in this chapter) and in most cases involves formation of a intracoronary thrombus occluding the coronary artery at the point of the accident (usually rupture of a plaque). The size of the infarction—in other words, the extent of the damage to the myocardium—depends on the size of the occluded coronary artery, the location of the occlusion, and the availability of protective collateral circulation. If irreversible ischemia affects a large portion of the muscle of the left ventricle, the patient may not survive; hence the first few hours after the initial chest pain are critical, and mortality in that period is high. However, ventricular fibrillation leading to cardiac arrest may also develop in patients with smaller infarctions; they can be successfully resuscitated and may completely recover.
The outlook for patients suffering a myocardial infarction who reach the hospital alive is favorable. Two studies dealing with early treatment, based on some 26,000 cases, showed that the survival rate for patients admitted to a hospital with myocardial infarction is
greater than 87 percent when they are treated conventionally and 90 percent when thrombolytic drugs are administered.
Diagnosis of Myocardial Infarction . A patient's description of an attack of chest pain and associated symptoms is often a sufficient basis for suspecting acute myocardial infarction. Further medical evaluation in the emergency unit, or later in the hospital, may confirm the diagnosis. Myocardial infarction can be classified by its size and location. The most extensive infarction is a transmural infarction , involving all layers of the wall of the left ventricle. It is also referred to as a Q-wave infarction because of certain electrocardiographic abnormalities. It can affect the front wall of the ventricle (anterior infarction) or the lower back wall (inferior infarction). This type of infarction causes the most serious damage and leads to the most complications. Subendocardial infarction affects the inner layer of the left ventricular muscle. Infarction of the right ventricle is less common than that of the left ventricle and as a rule represents an extension of an inferior infarction of the left ventricle. Myocardial infarction may also be less well defined, involving smaller sections of the myocardium, sometimes in more than one area. Furthermore, there are instances in which myocardial infarction can only be suspected, not proven.
The characteristic feature of the initial attack of myocardial infarction is chest pain, usually in the center of the chest behind the breastbone or across the upper portion of the front of the chest. Pain may radiate to one or both arms and the neck. The severity of the pain varies, but it develops without provocation and persists, usually unrelieved by nitroglycerin. About one-half of patients having their first myocardial infarction experience chest pain for the first time during that attack. In others it may be preceded by stable or unstable angina pectoris of varying duration. Other symptoms may accompany or follow the attack of chest pain—dyspnea, nausea with or without vomiting, pallor with cold perspiration, faintness or dizziness, and collapse.
Classic attacks of myocardial infarction are usually easy to identify, not only for a physician but also for the person stricken. There are many instances, however, when an attack may prove difficult to diagnose. Chest pain may be in unusual locations or so slight as to be dismissed by the patient. Occasionally pain is altogether absent;
in that case the patient may merely experience shortness of breath and sudden weakness or may collapse without warning. The reaction of someone experiencing a myocardial infarction runs from shock and alarm at recognizing the onset of a serious illness to dismissal of the pain as indigestion.
The course and outcome of acute myocardial infarction relates to many factors, the most important of which is the size of the infarct. Irreversible damage to a large portion of the left ventricle, the main pumping chamber, may be incompatible with survival. If the damage involves 40 percent or more of the left ventricular musculature, the outcome is invariably fatal: cardiac arrest soon follows, and efforts to resuscitate, even when immediate, are unsuccessful. Myocardial infarcts involving less than 40 percent of the muscle vary in their effect on the cardiac function according to the size and location of the infarct (those affecting the anterior wall are most serious); possible underlying heart disease, such as damage to the heart from hypertension or previous coronary episodes; the general condition of the patient and the presence of other diseases, such as diabetes, kidney disease, or lung disease; the presence of disease in other coronary arteries than the one occluded; and the development of complications.
The relationship between the size of the infarction and the effect on cardiac function, as modified by the various secondary influences, can be presented (in an admittedly oversimplified manner) as follows:
massive myocardial infarction® sudden death
very large infarction® cardiogenic shock
large infarction® left ventricular failure
small to medium infarction® no impairment of functions
This relationship describes the initial response of the heart to the sudden damaging effect of ischemia. The overall course is further determined by the secondary sequelae of the infarction and its possible complications. Yet the initial response to the attack is critical: about one-third of all deaths resulting from myocardial infarction occur immediately after the onset or within a few hours, often before the patient reaches a hospital. Out-of-hospital resuscitation of patients
can save only those with modest damage to the myocardium, that is, who have developed primary ventricular fibrillation.
Myocardial infarction produces a variety of secondary effects or complications. They most commonly develop within 48 hours of the attack and may or may not respond favorably to treatment. It should be reiterated, however, that close to 90 percent of patients admitted to the hospital survive the attack. Most enter the hospital after chest pain and associated symptoms have subsided and may feel well throughout the hospital stay. Yet their prognosis may be affected by one or more of the immediate sequelae:
Cardiogenic shock . This may develop with the initial attack, may come on gradually, or may strike suddenly later. Shock developing after a day or two of improvement is usually caused by a major complication of the infarction. Cardiogenic shock is associated with high mortality.
Arrhythmias . Ventricular arrhythmias are very common and are usually inconsequential if limited to premature beats. Monitoring of cardiac rhythm permits immediate intervention if more-serious ventricular arrhythmias develop. Atrial arrhythmias are less common and as a rule respond well to treatment.
Heart failure . This may be present at admission to the hospital or may develop later. Left ventricular failure requires immediate therapy but usually can be contained. Unresponsive heart failure, particularly if affecting both ventricles, is an unfavorable sign and often signifies an extension of the infarction to the right ventricle.
Hypotension . The patient's blood pressure is usually lower than normal following a heart attack; however, in some patients blood pressure falls below an acceptable level (but not low enough to cause shock). Appropriate treatment can rectify the problem.
Major complications of myocardial infarction include the following:
Extension of myocardial infarction . Occasionally a new attack of chest pain develops after one or two days without pain. New electrocardiographic abnormalities may show that the infarct has increased in size.
Angina pectoris . The patient may continue having attacks of chest pain after the initial attack has subsided, sometimes indicating disease in the nonoccluded branches.
Rupture of the heart . A transmural infarction may soften the infarcted muscle to the point that an opening develops connecting the ventricular chamber with the pericardial space. This complication is usually fatal, although in rare instances immediate surgery may save the patient's life.
Rupture of the ventricular septum . If the softened portion of the cardiac muscle affects the septum rather than the outside wall of the left ventricle, an opening between the left and right ventricles develops. In consequence, blood is shunted from the high-pressure left ventricle to the low-pressure right ventricle, greatly increasing the workload of the heart. Depending on the size of the opening, the result may be death, cardiogenic shock, or mild-to-moderate heart failure. Usually there is time to arrange for corrective surgery in more-serious cases. In milder cases surgery may be deferred until after recovery from the myocardial infarction.
Acute mitral regurgitation . Myocardial infarction occasionally damages one of the two papillary muscles in the left ventricle anchoring the mitral valve through the attached chordae. Such damage may produce incompetence of the mitral valve, leading to an overloading of the circulation. The effects of this complication are similar to those of rupture of the ventricular septum, and emergency surgery is often required.
Emboli in the systemic circulation . During the early stages of myocardial infarction clots may develop inside the left ventricle at the point where infarction may have damaged the endocardium. Portions of thrombi may detach themselves from the wall of the ventricle and travel in the bloodstream, producing emboli, which in turn may lead to stroke.
Pericarditis . Inflammation of the pericardium sometimes develops in the course of myocardial infarction. It is usually a benign complication and does not influence the course of the attack.
Heart block . Damage to the conducting system of the heart often produces varying degrees of conduction disturbance. This may be a transient phenomenon requiring no intervention. Often,
however, insertion of an electronic pacemaker is needed, either temporarily or permanently.
The diagnosis of myocardial infarction is based on three component findings—the initial attack of chest pain, a sequence of electrocardiographic changes, and the results of a blood test to determine the level of serum enzymes. The initial diagnostic evaluation is usually performed in an emergency unit. Great weight has to be placed on the patient's description of the attack since the physician's examination often contributes little to the diagnosis. The initial electrocardiogram may establish a tentative diagnosis of myocardial infarction, but full confirmation depends on two or more serially performed tests. Early testing for cardiac enzymes in the blood serum cannot contribute to the diagnosis because the characteristic rise in enzymes occurs several hours after the attack (peaking 12 to 24 hours later). Diagnostic difficulty may arise if the attack is atypical and the electrocardiographic changes are noncharacteristic or delayed. Since early treatment in an intensive-care unit is essential, patients suspected of suffering myocardial infarction are usually admitted to a hospital facility. The subsequent evaluation of doubtful cases, usually completed within 24 to 48 hours of hospitalization, can distinguish those patients whose chest pain is caused by abnormalities other than those of the heart or who are experiencing severe unstable angina without damage to the heart muscle.
The establishment of a diagnosis of myocardial infarction is only the first step in the diagnostic assessment of the problem. It is necessary to evaluate the damage to the heart and its consequences for the circulation and to recognize, or even anticipate, any change so as to provide appropriate treatment. The patient's heart rate and rhythm are continuously displayed on the cardiac monitor, the blood pressure is frequently checked, and a physical examination is performed periodically. In patients who are medically stable and feel well, observation and routine care may be all that is required. But further diagnostic procedures are available if indicated. A chest X-ray may show the presence of left ventricular failure. Echocardiographic examination or nuclide ventriculography permits an evaluation of the function of the damaged left ventricle, can provide information regarding the size and location of dead muscle tissue,
and can detect clots in the left ventricle. More elaborate tests may be needed if the patient's recovery is marred by continuing or delayed circulatory problems. Overt heart failure or shock may call for continuous monitoring of cardiac dynamics by means of flow-directed cardiac catheter. A complete cardiac catheterization and angiographic study may become necessary if a more severe complication is suspected.
Management of Myocardial Infarction . Acute myocardial infarction is a self-limited, self-healing disease of the heart that happens also to be a stage in coronary-artery disease. The goal of treatment is to facilitate the healing process, contain the damage to the heart, and protect the patient, if possible, from the sequelae and complications. In many cases the success of therapy is difficult to determine. The success of remedial interventions, such as treatment of heart failure or shock or surgical correction of some major complications, may be judged by the patient's response. But since most therapeutic endeavors are prophylactic, involving attempts to reduce the size of the infarct, prevent ventricular fibrillation, or avert major complications, their effectiveness in individual cases is not known. Evaluation of the success of therapy requires studies comparing results in large samples of treated and untreated patients. Interpretation of data from such intervention trials is often difficult, and the results are occasionally contradictory.
Acute myocardial infarction is in the majority of cases a benign disease. Many patients, even those suffering a large (transmural) myocardial infarction, feel well once the initial attack of chest pain has subsided and make an uneventful recovery. It is even possible to recover without any treatment. For example, occasionally an electrocardiogram taken during a routine checkup of a patient unaware of any heart problem shows unmistakable evidence that he or she has at some time suffered a major myocardial infarction. The initial attack in such a patient may have been milder than usual and have been overlooked or dismissed as an attack of indigestion; consequently the patient continued engaging in normal activity when he or she should have been treated in a hospital.
Nevertheless, the variability of the course of myocardial infarction, together with the possibility of major complications, makes it essential that once the condition is recognized appropriate management
be instituted. Treatment is usually initiated in an emergency unit or even during transport of the patient to the hospital. As soon as the probability of myocardial infarction has been established, the patient is moved to the coronary-care unit, first developed in the early 1960s. The expert care in this unit includes electrocardiographic monitoring of the patient's heart rhythm and hemodynamic monitoring in the event of circulatory failure.
Since the risk of serious arrhythmias is highest at the earliest stage of myocardial infarction, monitoring of cardiac rhythm is started as early as possible, in most cases in the ambulance transporting the patient to an emergency unit. Many ambulances are equipped with a transmitter to send electrocardiographic signals to the emergency unit, from where a physician can authorize and direct an ambulance attendant to administer drugs or use defibrillators.
The latest advance in the treatment of myocardial infarction is the use of thrombolytic drugs to dissolve the clot responsible for the infarction. The attack of chest pain usually indicates when a coronary artery became occluded. Death of heart muscle resulting from the occlusion occurs too rapidly to expect that dissolving the clot will prevent myocardial infarction. The basis for thrombolytic therapy rests in the hope that within the first few hours after the occlusion some myocardial cells are still capable of reviving if the blood supply is reestablished, thereby reducing the damage to the heart. Obviously, the earlier the drug is administered, the more likely the patient is to benefit from the treatment. Though this treatment is widely used, a small risk of serious hemorrhage leading to stroke or excessive blood loss calls for caution in administering it to certain patients. An alternative approach to early removal of coronary occlusion is interventional therapy (PTCA) performed immediately after diagnosis. Benefits of thrombolytic treatment have been demonstrated by two major studies, involving observations of thousands of patients, which have shown significant reduction of mortality from myocardial infarction. Benefits from PTCA have not yet been clearly demonstrated; hence its use is still considered experimental. Both approaches are subject to controversy and lively debate among experts regarding which patients are most likely to benefit from such intervention.
Following the initial intervention and after the patient has been moved to the coronary-care unit, management is guided by the
patient's condition. Those who feel well and show no significant abnormalities may be transferred within a day or two to a less intensive monitoring facility, then to a regular hospital room, and are candidates for early discharge. Patients who have arrhythmias, unstable blood pressure, or other abnormalities may require longer treatment in each type of facility. Dangerous manifestations, such as heart failure or shock, require intensive therapy, usually guided by hemodynamic monitoring by means of flow-directed cardiac catheters. Cardiogenic shock may require the use of an intraaortic balloon pump.
If chest pain continues beyond the first 24 hours or reappears along with evidence that its origin is ischemic, early coronary angiography may be performed. The results of that test may in turn indicate the need for coronary angioplasty or bypass surgery.
Serious complications of myocardial infarction tend to be delayed; most often they develop between the second and seventh days. Medical staff must be alert to complications at the earliest possible moment since the patient's life may depend on immediate intervention.
Fortunately, most patients will not experience such life-threatening complications and will feel well within 48 hours of the attack. At that point their chance of survival increases to 90–95 percent. The focus of medical management then shifts to rehabilitation. Cardiac rehabilitation in cases of myocardial infarction comprises supervising the patient's gradual resumption of activities and providing psychological support. Myocardial infarction often strikes active, healthy persons without warning, and the prospect of death or disability and uncertainty about the future may have a devastating effect on some patients. Most patients, however, are able to resume a life-style comparable to that prior to the attack, and in many the long-term prognosis is not affected by the attack. Two presidents of the United States, Dwight Eisenhower and Lyndon Johnson, were able to bear the immense stresses of the presidency after recovering from myocardial infarction.
A postinfarction survey of the status of the patient's circulatory system can be performed before discharge from the hospital or, as some physicians prefer, a few weeks later. Prognosis is related to several factors, which can be evaluated by tests: the presence or absence of ischemia as determined by an exercise stress test; the
function of the left ventricular pump as determined by echocardiogram or nuclide ventriculogram; and a tendency to precarious ventricular arrhythmias, as determined by a Holter monitor test. The precise battery of tests is tailored to the individual patient. They help the physician decide such questions as whether continuous drug treatment or some intervention (PTCA or bypass surgery) is needed.
Active persons convalescing from myocardial infarction must decide, under guidance from their physician, whether to resume their previous life-style without restrictions, return to their former occupation with some restrictions, retrain for a less strenuous or stressful occupation, or retire. All patients who recover from myocardial infarction should be encouraged to institute or continue preventive measures against atherosclerosis. This secondary prevention involves more aggressive modification of risk factors than does primary prevention. The main emphasis is on reducing cholesterol by diet and, if necessary, drugs. In many patients it is advisable to continue antianginal therapy or other forms of medical therapy.
Sudden Cardiac Death
Coronary-artery disease is the commonest cause of sudden cardiac death (see chap. 7). Sudden death may occur at any stage of coronary disease, but it is a particular concern during and immediately after the initial chest pain of myocardial infarction. In that context it may represent an electrical accident—primary ventricular fibrillation due to instability associated with severe but localized ischemia—or may result from ischemia involving such a large section of the left ventricular muscle as to make survival impossible.
Three patterns of sudden cardiac death (following the one-hour definition) occur in patients with coronary-artery disease:
instantaneous death without warning
cardiac arrest preceded by intermittent or continuous chest pain
cardiac arrest preceded by severe dyspnea including pulmonary edema
Primary ventricular fibrillation is by far the most frequent mechanism of cardiac arrest in these cases. Such patients can usually be
resuscitated, and the rate of survival and recovery is good. This response has led some communities to develop programs of widespread training of citizens in cardiopulmonary resuscitation (CPR) for keeping alive someone suffering a heart attack until appropriately equipped medical personnel arrive. Many people have been resuscitated outside the hospital by such means. Studies of survivors of resuscitation reveal that more than half suffered cardiac arrest during the initial stage of myocardial infarction. Among other survivors the arrest was caused by primary ventricular fibrillation resulting from reversible ischemia or was noncoronary in origin. Patients in later stages of coronary-artery disease, particularly those with pump failure, are more difficult to resuscitate and, if successfully resuscitated, have a poor chance of long-term survival.
The most perplexing problem concerns the person with no prior symptoms of coronary-artery disease in whom sudden cardiac death is its first manifestation. Some researchers have attempted to determine whether such apparently healthy persons did indeed have some warning they ignored. Interviews with the families of the deceased revealed that quite a few had sought medical care a short time before the fatal episode. However, symptoms suggesting coronary-artery disease were not always recorded. Since significant symptoms are often ignored, misinterpreted, or subconsciously suppressed, it is possible that warning signs precede sudden cardiac death more often than generally suspected. The available interventions aimed at preventing ventricular fibrillation are limited, yet in a hospital there is a reasonable chance of reviving someone from cardiac arrest—hence the importance of seeking medical care for coronary-artery disease as early as possible.
Chronic Pump Failure
Chronic pump failure, a late stage of coronary-artery disease, results from the destruction of myocardial cells by the recurrent ischemia. In many cases ischemic chest pain is no longer present. Four mechanisms may produce chronic pump failure. (1) A large, transmural myocardial infarction (often leading to left ventricular aneurysm) may affect cardiac function despite survival and recovery from cardiac arrest, so that heart failure persists. (2) Multiple attacks of myocardial infarction can gradually destroy enough heart
muscle to cause chronic cardiac failure. (3) Small areas of ischemic damage to the heart muscle, regardless of the presence of myocardial infarction or angina pectoris, may cause heart failure imitating cardiomyopathy (see chap. 10). (4) Mitral insufficiency produced during myocardial infarction may overload the circulation and cause chronic heart failure.
The first two mechanisms account for most cases of chronic pump failure. The actual impairment of cardiac performance does not necessarily determine the amount of disability caused by the heart failure. Despite a low ejection fraction indicative of serious damage, the patient may continue a life free from symptoms, particularly if his or her life-style does not involve strenuous activity. Gradual resumption of activities after myocardial infarction and caution exercised thereafter may prevent the development of dyspnea and other manifestations of heart failure. Nevertheless, significant impairment of cardiac function indicates potential problems even if symptoms are minimal or absent, and the prognosis in such cases is guarded, particularly since patients with impaired left ventricular function are prone to life-threatening ventricular arrhythmias.
Symptomatic patients with chronic pump failure due to coronary-artery disease respond to standard therapy, which may control disability for long periods, especially if the patient is free from angina pectoris. A stable functional impairment of cardiac function without further evidence of ischemia may, with optimal medical management, permit a long life with only a minor effect on its quality. Many other patients, however, suffer from serious disability, developing major complications and even end-stage heart failure, the only remedy for which is cardiac transplantation.