1.2—
The Molecular Structure of Plant Cell Walls
Polysaccharides are the principal components of all plant cell walls. The polysaccharides of the cell wall are made up of sugars which are linked to each other by glycosidic bonds to form the polymer chains. Each polysaccharide contains particular kinds of sugars which are joined to each other in characteristic patterns of linkage position and sequence. It is now known that the secondary, tertiary and quaternary structures of cell wall polysaccharides are determined by the structures of the component sugars and the linkages between them, just as the three dimensional structure of a protein is determined by the sequence and structures of its component amino acids (Rees, 1972).
The various polysaccharide chains of the plant cell wall are connected to each other in specific ways, and they form an integrated network. The properties of this network depend not only on the amounts, characteristic properties and orientations of the individual polysaccharides, but also on the nature and frequency of the interconnecting linkages between them.
The conformational structures of the nine sugars commonly found in plant cell walls are shown in Fig. 1.1. The three types of polysaccharide normally found in plant cell walls (cellulose, hemicelluloses, and pectic polysaccharides), and the structural protein of primary walls, are described briefly below.
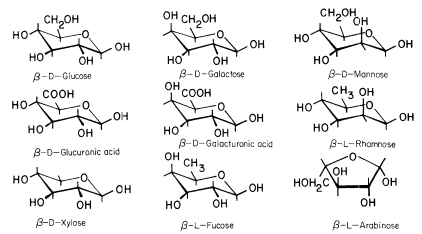
Figure 1.1
Sugars of plant cell walls.
Conformational line drawings indicate approximate bond angles. b -L arabinose is shown in its
preferred planar furanose ring form. The other sugars are shown in their most stable pyranose
chair form. Carbon atoms are numbered as indicated for b -D -glucose. Ring hydrogens are
indicated by bonds only. Note that groups attached to a ring may be either axial (projecting
above or below the ring) or equatorial (projecting to the side of the ring). Substituents at C1
project equatorially in the b configuration, but are axial in the a configuration. All 'bulky'
groups (–OH, –CH2 OH, & –COOH) are in equatorial positions in b -D -glucose, b -D -
glucuronic acid, and b -D-xylose. Note that these sugars differ only in the group
attached to C5 . Galactose, galacturonic acid and fucose are similarly related
(axial –OH group at C4 ), as are mannose and rhamnose (axial –OH group at C2 ).
1.2.1—
Cellulose
Cellulose occurs as a crystalline, fibrillar aggregate of b -1,4-linked glucan chains (Frey-Wyssling, 1969). Cellulose fibrils give plant cell walls most of their enormous strength, much as glass fibres embedded in an epoxy resin give strength to a fibreglass composite (Northcote, 1972).
The basic structure of the b -1,4-linked glucan chains of cellulose is illustrated in Fig. 1.2 by conformational line drawings and in Fig. 1.3 by molecular models. Residues of b -D -glucose (Fig. 1.1) are glycosidically linked to each other, from carbon 1 of one residue to carbon 4 of the adjacent residue. The upside-down inversion of every second residue in the chain minimizes contact between atoms of adjacent residues. Close inspection of the models in Fig. 1.3 shows that the –OH groups at carbon 3 are in very close proximity to the ring oxygens (O5 ) of adjacent residues. Hydrogen bonds between O3 and O'5 help to stabilize the flat, straight, ribbon-like structure of b -1,4-linked glucan chains.
The flat, ribbon-like structure allows the chains to fit closely together, one on top of the other, over their entire lengths. These interchain associations are stabilized by hydrogen bonds between O6 of a glucose residue in one chain and
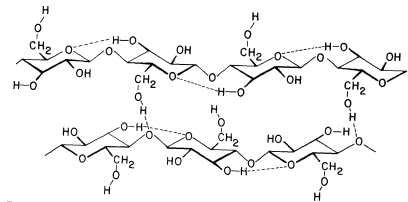
Figure 1.2
b -1,4–linked glucan chains of cellulose.
Portions of two associated chains are illustrated by
conformational line drawings. Distances between atoms
are not accurately indicated in this illustration, but see Fig. 1.3.
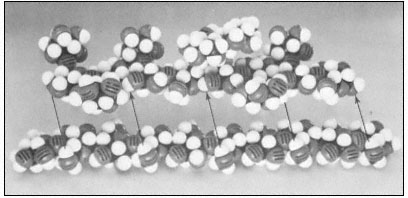
Figure 1.3
Hemicellulosic xyloglucan associated with cellulose.
The repeating subunit of a hemicellulosic xyloglucan is shown in
association with a portion of a b –1,4 –linked glucan chain of cellulose
(Bauer et al., 1973). Molecular models have been used to accurately indicate
interatomic distances and bond angles. Hydrogen bonds from the cellulosic
glucan chain to the glucan backbone of the hemicellulose are indicated by arrows.
the oxygen of the glycosidic bond (O1 ) between glucose residues in an adjacent chain. Since the glucan chains of cellulose are very long (8,000 to 15,000 residues), the number of hydrogen bonds between adjacent chains is very large. The resultant crystal is extremely stable and so tightly packed that there is no room for water molecules in the crystal structure.
Although there is some controversy as to whether native cellulose fibrils are 3.5 nm or 10 nm in diameter, it is clear that the glucan chains of cellulose
aggregate to form stiff crystalline rods of very considerable length and mechanical strength. The molecular structure of b -1 ,4-linked glucose thus neatly determines the secondary and tertiary structures of cellulosic glucan chains, and establishes the quaternary, interchain associations—although not the dimensions—of the microfibrils. The stiff, crystalline rods of cellulose are clearly well suited to their biological function in the plant cell wall.
1.2.2—
Hemicelluloses
Xylans, arabinoxylans, galactomannans, glucomannans and xyloglucans are common types of hemicelluloses (Timell, 1965). The basic repeating sequence of a hemicellulosic xyloglucan molecule is shown in Fig. 1.3, adjacent to a cellulosic glucan chain.
Although different hemicelluloses have different component sugars, all have two structural features in common which bear importantly on their biological function. (1) All hemicelluloses have straight, flat b -1,4-linked backbones. Any side chains attached to the backbone are short—usually just one sugar long—and stick out to the sides of the backbone (cf. Fig. 1.3). (2) All hemicelluloses have some structural feature which prevents the chains from extended self-aggregation of the type which exists between the b -1,4-linked glucan chains of cellulose. Xyloglucans, for example, have a b -1,4-linked glucan backbone (just as cellulose), but most of the glucose –CH2 OH groups (C6 ) are substituted with xylose side chains (see Fig. 1.3) and are thus not available for the formation of interchain hydrogen bonds. Similarly, since xylose is a pentose (i.e. a five carbon sugar), the b -1,4-linked xylose backbones of xylans and arabinoxylans have no–CH2 OH groups available for interchain hydrogen bonding. The glucomannans have b -1,4-linked backbones containing both glucose and mannose. The –CH2 OH groups of these sugars are unsubstituted, but the axial conformation of the –OH groups at carbon 2 of the mannose residues (cf. Fig. 1.1) prevent close interchain associations wherever mannose residues occur in the backbone.
Although hemicelluloses cannot self-aggregate to form long, close-packed crystalline fibrils in the manner of cellulose, the chains of hemicellulosic polysaccharides can form important hydrogen bonded associations with each other in the cell wall, particularly between regions of the chains which have few side branches or axial hydroxyl groups (Blake & Richards, 1971; McNiel et al., 1974). Even more importantly, hemicelluloses can co-crystallize with cellulosic glucan chains at the surface of the cellulose microfibrils (Bauer et al., 1973, Northcote, 1972). The cocrystallization probably involves the formation of hydrogen bonds from the –CH2 OH groups present in cellulose chains to the glycosidic oxygens in the adjacent hemicellulose chains (see Fig. 1.3). This association would form a tightly bound monolayer of the hemicellulose on the surface of the cellulose microfibril, and would function as part of the 'glue' which holds the microfibrils together in the cell wall.
1.2.3—
The Pectic Polysaccharides and Structural Protein
1.2.3.1—
Rhamnogalacturonans
The rhamnoglacturonans are long polymers of a -1,4-linked galacturonic acid interspersed with a few residues of 1,2-linked rhamnose (Aspinall, 1973). There is some evidence that the rhamnosyl residues may occur at definite positions in the galacturonan chain, giving a subunit structure to the rhamnogalacturonan polymer (Talmadge et al., 1973; see Fig. 1.4).
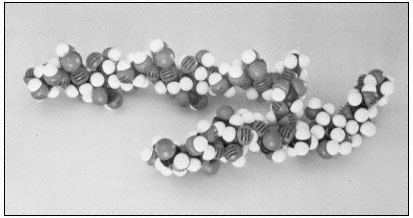
Figure 1.4
Pectic rhamnogalacturonan.
CPK models illustrate the repeating subunit of a rhamnogalacturonan,
with a short sequence of b -1,4-linked galactan attached to C4 of one
of the rhamnosyl residues (Talmadge et al., 1973). The sequence
of the subunit is GalUA8 Rha GalUA Rha GalUA4 .
The diaxial conformation of the a -1,4 glycosidic linkages between galacturonic acid residues (see Fig. 1.1) causes the orientation of adjacent rings to be twisted. Thus, the galacturonan polymer forms a tight, stiff, rod-like helix with three residues per turn (Rees & Wight, 1971; Fig. 1.4). The insertion of 1,2-linked rhamnosyl residues in the galacturonan chain creates 'kinks' or right angle bends.
Divalent cations, particularly calcium, form complexes with the carboxyl and hydroxyl groups of galacturonic acid residues in the polymer. Complex formation of this type occurs primarily between adjacent residues in a galacturonan polymer, but could serve to create ionic ligand bridges between adjacent galacturonan chains.
1.2.3.2—
Arabinogalactans
Two distinct types of arabinogalactans are known to occur in plant cell walls (Aspinall, 1973). The first type has a b -1,4-linked galactan backbone with
highly branched arabinose side chains. The second type has a b -1,3-linked galactan backbone with many short side chains containing galactose and arabinose.
1.2.3.3—
Structural Protein
Primary cells walls (i.e. the type of walls characteristic of actively growing cells) contain a structural protein component. While the structural protein of these walls has not yet been isolated as an intact molecule, the analysis of peptide fragments from the primary walls of dicotyledonous plants has revealed several interesting characteristics (Lamport, 1970; Lamport et al., 1973). This structural protein contains over 25% hydroxyproline, which is an unusual amino acid known to break the continuity of a -helical structures. In animals, hydroxyproline occurs almost exclusively in the proteins of connective tissue (collagen and gelatin). Several tryptic peptides of the structural protein have been isolated,
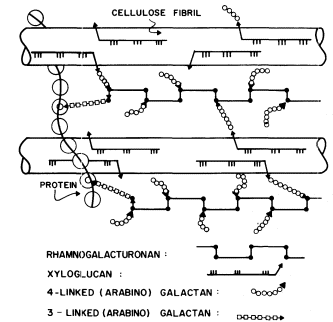
Figure 1.5
Interconnections between cell wall components.
Schematic representation of the polymeric components of
sycamore primary cell walls and their interconnections (Keegstra et al.,
1973). Hemicellulosic xyloglucan polymers are cocrystalized with cellulosic
glucan chains on the surface of the (two) microfibrils. The reducing ends of some
—but not all—of the xyloglucan chains are glycosidically attached to some—but not
all—of the b -1,4-linked (arabino) galactan side chains of the rhamnogalacturonan polymers.
The rhamnogalacturonan polymers and the structural protein are interconnected by 1,3-linked
(arabino) galactan bridges. Arrowheads indicate the reducing ends of polysaccharide chains.
and all appear to contain the sequence Ser-Hyp4 . Arabinose tetrasaccharides are glycosidically linked to virtually all of the hydroxyproline residues in the protein. In addition, many of the serine residues are glycosylated with galactose. As a result of this extensive glycosylation, the structural protein is very resistant to degradation by proteases, and is likely to have an extended, rod-like shape.