IV—
Research and Development, 1932–36
1—
A Fruitful Error
A Claim
The Laboratory's first sustained appearance on the international scene was the result of mixing heavy water with resonance acceleration. The mix gave rise to the false idea, which Lawrence defended resourcefully, that the deuteron is unstable. This hypothesis of deuteron disintegration had many unpleasant implications for nuclear theory. It therefore immediately excited incredulity and opposition in the wider world of physics. The persistence of the Laboratory in its error spread its reputation for sloppiness and forced others to make an important discovery; and it illustrated the strengths and the weakness of the Laboratory's style of teamwork centered entirely on Lawrence's research program.
Lewis and Lawrence first loosed deuterons into Livingston's 27-inch cyclotron in April 1933. Their expectation that "heavy protons" would be better projectiles than light ones was quickly fulfilled.[1] On May 3 Lawrence wrote his close colleagues—Boyce, Cooksey, and Oppenheimer—that "H-twotrons" blasted lithium into alpha particles with ranges in air of 8.2 cm and 14.8 cm, and knocked slow alpha particles from boron, magnesium, nitrogen,
[1] Lawrence to Poillon, 14 Apr 1933 (15/16A). The deuterons, at 1.3 MeV, came from H H ions accelerated to 2 MeV; Lewis, Livingston, and Lawrence, PR, 44 (1 Jul 1933), 55 (letter of 10 June).
and aluminum. The yield from lithium was ten times larger with deuterons than with protons. Lawrence hurried to capitalize on the new projectile and the new machine between the end of the term and early June, when he was to leave for vacation and teaching at Cornell. "I have been working night and day in the laboratory since class ended," he wrote Oppenheimer. "You will pardon me for dictating this. I am doing it while proctoring an examination. I am really trying to conserve time."[2]
Explaining the action of deuterons on lithium to Cockcroft, Lawrence supposed that the swifter alpha particles came from Li7 , according to Li(d,n)2a , and the slower from Li6 via Li(d)2a . A week later he changed his mind and identified the fast alpha particles with the disintegration of Li6 . Both processes, whichever was which, went well even with deuterons of modest energy. To coax alpha particles from aluminum, magnesium, and nitrogen, however, required energies above a million volts, well out of Cockcroft's range.[3] Every element bombarded with deuterons of such energies "disintegrated." It was time for a news release. The release: lithium, beryllium, boron, nitrogen, fluorine, aluminum, and sodium all suffered "transitions" at Berkeley; "at this rate of progress, one dares not guess what will be achieved in nuclear physics within a few years."[4] Within a few weeks the rate was retrograde; Lawrence still could not say for certain from which lithium the fast alpha particles came, nor whether anything else but nitrogen, and possibly beryllium and boron, disintegrated under bombardment by deuterons of 1.3 MeV.[5]
Then came an extraordinary find. Not stopping to resolve the problems they had raised, the Berkeley group found that every element gave off protons under bombardment by deuterons with more than 800 keV of energy. And more than that: the protons all
[2] Lawrence to Boyce (3/8), Cooksey (4/19), and Oppenheimer (14/9), all 3 May 1933; Lawrence to Boyce, 2 Feb 1933 (3/8), for Lawrence's summer plans.
[3] Lawrence to Cockcroft, 4 May 1933 (5/4); to Darrow, 10 May 1933 (6/9).
[4] Lawrence to Houtermanns, 12 May 1933 (8/11); Science service , 20 May 1933 (Lewis P), quote.
[5] Lewis, Livingston, and Lawrence, PR, 44 (1 Jul 1933), 55–6 (letter of 10 June), 317 (abstract for June meeting of the APS); Henderson also participated in the work. Cf. Lawrence to Cooksey, 2 June 1933 (5/4).
had the same range, 18 cm, irrespective of the material from which they came. "I am almost bewildered by the results," Lawrence wrote. But only almost. He reasoned that such homogeneous protons could scarcely come from so heterogeneous a set of elements, especially from the highly repulsive (to deuterons) nuclear charges of atoms as heavy as gold; and he declared that they must originate not in the targets but in the projectiles. "We have strong evidence that we have disintegrated the deuteron itself." So Lawrence wrote Bainbridge, asking for his latest values of the masses of H2 by return airmail.[6] The numbers were needed to calculate the mass of the neutron from the hypothesis of deuteron disintegration.
In the easiest hypothetical case, a deuteron of energy 1.33 MeV hits a gold nucleus, which is too heavy to be moved much by the blow and splits into an 18-cm proton and a neutron of unknown velocity. Lawrence's group had some shaky evidence that in the explosion of the deuteron, the constituents received equal momentum and energy. Now an 18-cm proton has an energy of 3.6 MeV; the kinetic energy produced in the explosion is accordingly (7.20 – 1.33)MeV = 0.0063 mass units. This, together with the masses of the proton and the neutron, must equal the mass of the deuteron, which Bainbridge had fixed at 2.0126 from his analysis of Lewis's water. The indicated arithmetic produced the startling answer, mn» 1.000. Thus was born the short-lived light Berkeley neutron.[7]
Lawrence's idea may be reconstructed from an improved calculation made after his return from Cornell and from later correspondence. The calculation rested on experiments by Henderson and Livingston, which seemed to show that the proton came off with all the kinetic energy of the deuteron and that a bombarding energy of 1.2 MeV, not 1.33 MeV, was enough to give protons of 18 cm. Therefore, in the explosion the proton gets 3.6 – 1.2 = 2.4 MeV and, to conserve momentum, the neutron does too. (The new numbers raised mn slightly, to 1.0006). The
[6] Quotes from, resp., Lawrence to Cockcroft, 2 June 1933 (5/4), and to Bainbridge, 3 June 1933 (2/20).
[7] Lawrence, Livingston, and Lewis, PR, 44 (1 Jul 1933), 56 (letter of 10 June); Bainbridge, ibid., 57 (letter of 14 June).
model: the deuteron tends to explode in the field of a nucleus at the place where it experiences the strongest force for the longest time, at rest at its closest approach. The neutron and proton receive equal and opposite momenta; in addition, the charged proton is pushed out by the electrostatic force of the nucleus, which returns to it the energy lost by the parent deuteron in penetrating to the place of its demise.[8]
Lawrence advertised the light neutron at a symposium held at Caltech in May 1933 in honor of Bohr. Always hoping for revolution, Bohr welcomed Lawrence's news as a "marvellous advancement." Millikan, the master of self-advertisment, praised Lawrence's work as "altogether extraordinary, and most intelligently announced."[9] On the way to Cornell Lawrence stopped at the Century of Progress Exposition in Chicago, where the American Physical Society held, on its own assessment, "perhaps the most important scientific session in its history." Aston, Bohr, Cockcroft, and Fermi, among others, were there as guests of the AAAS to help celebrate. In a special session on nuclear disintegration, Cockcroft discussed the blasting of light elements by his voltage multiplier, Tuve described the blasting of middling elements by his Van de Graaff generator, and Lawrence claimed the blasting of everything by the cyclotron. Bohr presented a public commentary, during which he wrapped himself up in the wave-particle duality and the microphone cord. It was not a hard act to follow. "It was much easier, and much more pleasant [wrote the reporter from Time ] to understand round-faced young professor . . . Lawrence . . . tell how he transmuted elements with deuton bullets."[10]
Lawrence stole the show. The New York Times 's correspondent, William L. Laurence, introduced his namesake as the proprietor of a "new miracle worker of science," which, when whirling deuterons, liberated about ten times the energy it was fed. "The newest developments give only an inkling of what lies in store for man when and if he finally succeeds in unlocking what
[8] Livingston, Henderson, and Lawrence, PR, 44 (1 Nov 1933), 781–2 (letter of 7 Oct); Darrow to Lawrence, 28 Nov 1933, and answer, 9 Dec 1933 (6/9); Boyce to Lawrence, 19 Feb 1934, and answer, 27 Feb 1934 (3/8).
[9] Childs, Genius , 199.
[10] PR, 44 (1933), 313–4; "Complementarity in Chicago," Time, 22 (3 Jul 1933), 40.
Sir Arthur Eddington calls the 'cosmic cupboard of energy'."[11] Lawrence explained that the cracking of the deuteron released a little of that vast store of atomic energy of which Aston had given an inkling in his famous overstatement that half a glass of water could drive the Mauretania across the Atlantic. According to the Laboratory's measurements and theories, the neutron weighed less than the sum of the weights of the proton and the electron, which Lawrence understood to be its constituents. Hence, according to the relativistic equivalence of mass and energy, the combination of a proton and an electron should yield energy. Here was another source—along with the disintegrating deuteron—of perpetual motion.[12] Lawrence did not bother readers of the Times with the worry that hydrogen atoms might, or rather should, collapse spontaneously to neutrons and blow the world apart.
A Doubt
Meanwhile the Cavendish Laboratory had started to project deuterons. Rutherford had been most eager to procure heavy water for his experiments even before the startling discovery of the efficacy of deuteron bombardment. When Fowler returned to Cambridge from Berkeley early in May 1933 without any, he was "nearly lynched," he told Lewis, who forthwith forwarded a protective ampule containing as much deuterium as Lawrence's group had used in their disintegration experiments. Rutherford promised through Fowler not to hurry any results he might obtain into print, "so as to give Lawrence plenty of chance to get in first."[13] He received the news that deuterons had smashed atoms of lithium, nitrogen, magnesium, and beryllium. It reminded him of better times and of the Maori warrior on his baronial shield. "I should like to congratulate Lawrence and his colleagues for the
[11] Seidel, Physics research , 381, from William L. Laurence, "New 'gun' speeds breakup of atom: 'Deuton's bullet frees ten times its own energy,' scientists are told," New York Times (20 June 1933), 1. By our count the factor was five (7.2/1.33) for the deuterons that disintegrated; it is, of course, scarcely worth mentioning in comparison with Laurence's exaggerations.
[12] Laurence, "Neutron 'weighed' by Prof. E.O. Lawrence, proves lighter than its component parts," New York Times (24 June 1933), 1.
[13] Fowler to Lewis, 9 May 1933, and Lewis to Rutherford, 15 May 1933 (Lewis P).
prompt use they have made of the new club to attack the nuclear enemy. . . . These developments make me feel quite young again."[14]
Rutherford hurled his clubs from a proton accelerator that had been made for him by Oliphant to follow up the experiments of Cockcroft and Walton. This accelerator may stand as a symbol of Rutherford's methods in contrast with Lawrence's. Rather than go to higher energies than Cockcroft and Walton had reached, Rutherford opted for lower; theirs could attain 800 kV, his only 200 kV. He wanted to examine the thresholds of proton-induced nuclear reactions, identify the products, and estimate yields as functions of the energy of bombardment. To achieve his purposes, he directed that his new machine have a large proton current; a directive so well executed that Oliphant and Rutherford had for their experiments about 1,000 times the single microamp of Cockcroft and Walton's early experiments.[15]
The Oliphant-Rutherford accelerator, though short on energy, was by no means the cheap, jerry-rigged contraption of string and sealing wax dear to Cavendish mythology.[16] Metropolitan-Vickers designed, built, and contributed the oil-diffusion pump that created the vacuum, which was produced and maintained with the help of Apiezon oils and greases; the accelerating system incorporated the voltage-multiplier circuit perfected by Cockcroft and Walton with the help of Metro-Vick's engineers and a 100-kV transformer bought from Metro-Vick at what Rutherford thought the extravagant price of 85 pounds; and the detectors, which recorded the ionization created in a special chamber by the disintegration products, used a linear amplifier, thyratron tubes, and a purely electronic counting system then just invented at the Cavendish by C.E. Wynn-Williams and his co-workers.[17] The Cavendish was then far ahead of Berkeley in electronics and vacuum technology and in integrating the work of academic
[14] Rutherford to Lewis, 30 May 1933 (Lewis P).
[15] Rutherford to Hevesy, 3 Apr 1933, in Eve, Rutherford , 370; Oliphant and Rutherford, PRS, A141 (1933), 259–81, in Rutherford, CP, 3 , 329–50.
[16] Crowther, Cavendish , 242–4, and Cockburn and Ellyard, Oliphant , 49–50, both relying on Oliphant.
[17] Oliphant and Rutherford, PRS, A141 (1933), in Rutherford, CP, 3 , 330–4; Allibone in Hendry, Cambridge physics , 158–9, 161–2, 169; Wynn-Williams in ibid., 142–7; Hendry, ibid., 114–9.
physicists and industrial engineers.
By February 1933 Oliphant and Rutherford were extending Cockcroft and Walton's results, provoking the disintegration of lithium with protons of only 100 kV. "It is a great show! But who would have thought that anything would happen at 100,000 volts, except perhaps Rutherford?"[18] In June, about the time the heavy water came to hand, they presented an account of their work on proton disintegration. They found that lithium's threshold stood at 20 kV and boron's at 60 kV; that beryllium's could not be determined because of its very small yield; and that in elements heavier than boron, except for a trace at fluorine, even the most energetic protons available, at 200 kV, did not stimulate disintegration. Oliphant and Rutherford traced apparent reactions in heavy metals such as gold to disintegration of boron impurities from the glass walls of their pyrex discharge tube.[19] This was an important warning. Lawrence did not heed it: he was too busy running through the periodic table, too eager to accept the astounding, to take the time to track down subtle effects.
When he ran Lewis's water against lithium, Oliphant detected particles of 13.2-cm range, which he identified with Berkeley's particles of 14.8 cm. "The Professor seems very happy."[20] Soon Oliphant and Rutherford confirmed the existence of rays that penetrated to 8.2 cm, which they showed to be alpha particles with the maximum energy possible in the reaction Li7 (d,n)2a .[21] Walton informed Cockcroft, then visting the Laboratory, of the good general agreement of Cambridge's results with Berkeley's. Cockcroft found himself curiously placed: he sat in the camp of one of his competitors while his partner, Walton, sat in the other. He decided that the most attractive subject for them was the prolific 18-cm proton, which Oliphant and Rutherford could not excite and Lawrence's company could not stop to study. "We ought to be able to get many of these [Cockcroft advised Walton]
[18] Fowler to Bohr, 15 Feb [1933], in Hendry, Cambridge physics , 107.
[19] Oliphant and Rutherford, PRS, A141 (1933), in Rutherford, CP, 3 , 335–7, 343, 349–50; Rutherford to Hevesy, 3 Apr 1933, in Eve, Rutherford , 370.
[20] Dee to Cockcroft, 10 Jun 1933 (CKFT, 20/4), quote; Rutherford to Boyle, 28 Jul 1933, in Eve, Rutherford , 374.
[21] Dee to Cockcroft, 7 Jul 1933 (CKFT, 20/7); Oliphant, Kinsey, and Rutherford, PRS, A141 (1933), 722–33, in Rutherford, CP, 3 , 354–6, 358–60.
and I hope you will be able to [borrow] some of the Professor's
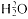
The Cavendish work, and Rutherford's congratulations to Lawrence on the "fine reward for his labour in developing his accelerated [!] system," pleased the Berkeley group and probably helped to harden their belief in what Lewis called "the most important discovery so far[:] the essential instability of the H2 nucleus and the low mass of the neutron." Cockcroft was also pleased at the confirmation obtained using the Oliphant-Rutherford accelerator. That had given him the hope that with the Cockcroft-Walton machine it would be possible to detect the 18-cm protons, for which Lawrence gave a threshold of 700 kV. "If so [Cockcroft wrote Rutherford] it [sic] will find a whole lot more work to be done with the present apparatus."[23]
To make all this work possible, the Cavendish required a steady supply of heavy water. Rutherford detailed a visitor, Paul Harteck, from the Kaiser-Wilhelm-Institut für Chemie, to the task. The Cavendish apprentice system in this respect paralleled Berkeley's: Harteck had been told to help with electronic counters, but was reassigned because of his knowledge of chemistry when Rutherford decided to domesticate the manufacture of deuterium. The change did not please Harteck. "I must take on the production of heavy water at the wish of the high Lord," he wrote his patron, K.F. Bonhoeffer. "If you know anything [about it], write me soon, for with the Lord everything must go very quickly. . . . You must hurry, since heavy water evidently seems to be no rarity in America."[24] The gift from Lewis took the heat off Harteck, who had not succeeded. An application to Lewis for information elicted a full answer that did not help; and by the end of June,
[22] Walton to Cockcroft, 20 June 193[3] (CKFT, 20/35); Cockcroft to Walton [24 June 1933] (ER).
[23] Lewis to Rutherford, 12 Jul 1933, and Cockcroft to Rutherford, 22 Jul 1933 (ER).
[24] Harteck to Bonhoeffer, 28 Apr and 12 May 1933 (Harteck P). Cf. Harteck and Streibel, Zs. anorg. und allgem. Chemie, 194 (1930), 299.
Harteck could only imagine that there was some trick to the business that had been withheld from him. Rutherford then lost half the original sample. Had Lewis then turned off his water, the Cavendish deuteronomers would have been out of business until the end of October, when Harteck managed to make enough heavy water at sufficient concentrations for their needs.[25]
There was already good reason to worry that Berkeley water did not give the same results in England as at home. Walton and Dee saw only a few 18-cm protons, nothing like the profusion the Berkeley group had reported. "I noticed Lawrence's views about the nature of these tracks," Rutherford wrote Lewis at the end of July, "but we are at the moment not inclined to view with favour the conversion of a deuton into a neutron of mass about 1. However, it is too early to take definite views."[26] The principal parties then relaxed for the summer, to prepare for more definite views in the fall.
The Error
At Berkeley preparation included improvements in the cyclotron, which made possible production of 0.02 µA of 3 MeV deuterons, and curing "vacuum troubles and other misfortunes" that kept the machine down for most of September.[27] When the streams began to flow, they called forth showers of neutrons from everything they hit, a confirmation most agreeable to the minds, but also threatening to the bodies, of the cyclotroneers.[28] (The Laboratory was so full of stray neutrons that an investigator quirky enough to have tested the fillings in his teeth might have discovered artificial radioactivity.) Another set of doubters then
[25] Harteck to Lewis, 4 June, and Lewis to Harteck, 23 June 1933 (Lewis P); Harteck to Bonhoeffer, 9 and 28 June 1933 (Harteck P); Rutherford to Lewis, 27 Jul, 10 Aug, 21 and 30 Oct 1933 (Lewis P); Cockcroft to Rutherford, 23 Aug and 29 Sep 1933 (ER). Imperial Chemical Industries had trouble with the plant it built not long after Harteck got his first heavy droplets; Fowler to Lewis, 3 Feb 1934 (Lewis P).
[26] Darrow to Lawrence, 11 Oct 1933, Livingston to Darrow, 20 Oct 1933, and Lawrence to Darrow, 20 Nov 1933 (6/9); Rutherford to Lewis, 27 Jul 1933 (Lewis P).
[27] Lawrence to Cooksey (5/4) and to Tuve (3/32), 23 Sep 1933.
[28] Lawrence to Barton, 28 Sep 1933 (2/25), to Beams, 4 Oct 1933 (2/26), and to Tuve, 9 Oct 1933 (3/32); Lewis to Rutherford, 5 Oct 1933 (ER).
entered the game. Richard Crane, a junior collaborator of Lauritsen's at Caltech, came to Berkeley, collected some heavy water, dribbled it on a beryllium target in Lauritsen's machine, and got "enormous quantities of neutrons." That was of course most gratifying, "in entire agreement with our expectations," Lawrence wrote Cockcroft, "though the precise interpretation is as yet ambiguous." The point of imprecision was whether Crane's neutrons came from the disintegration of the beryllium target or of the deuteron projectile. In either case, however, Lawrence thought that Crane's evidence favored a value of the neutron mass close to unity.[29]
Once the cyclotron returned to work, Livingston and Henderson found quantitative evidence of the deuteron's instability. They counted the number of "disintegration" protons (some 40,000 per minute registered in their ionization chamber) and then the number of "recoil" protons reported by the same chamber when covered with a wax-coated lead screen (12/min.). Their previous estimate of the probability of the conversion of neutrons to protons in wax suggested that 40,000 neutrons would make around 12 protons. Hence neutrons and protons appeared in equal numbers, which would be necessary if they came from the breakup of deuterons. They realized that this agreeable agreement had "profound theoretical implications" through its relevance to the value of the neutron mass, which they set at 1.0006. Their report, signed also by Lawrence, appeared on November 1.[30]
At Cambridge preparation included completing reports for the Solvay conference to be held in Brussels at the end of October. Cockcroft had responsibility for reviewing particle accelerators and Chadwick for the state of knowledge about neutrons. Lawrence contributed by sending Cockcroft information about the cyclotron and by bringing his latest evidence for disintegration to the Solvay meeting in person. The invitation to attend, at his own expense for travel, was a great honor; Lawrence was but the eighth American so distinguished since the conferences began in 1911
[29] Quotes from, resp., Lawrence to Tuve (3/32) and to Cockcroft (5/4), 23 Sep 1933.
[30] Livingston, Henderson, and Lawrence, PR, 44 (1933), 782; Lawrence to Tuve, 9 Oct 1933 (3/32); Lawrence to R.C. Gibbs, 9 Feb 1933 (9/16), on Henderson.
and the only one in 1933. He declared himself "surprized and tremendously pleased,"[31] though the invitation came very late, some six weeks before the meeting (he owed it to Peter Debye, a member of the Solvay scientific committee, who had visited Berkeley the previous summer and taken a fancy to the Sloan x-ray tube).[32] Lawrence went to this most august of physicists' gatherings (fig. 4.1), the first international meeting he had ever addressed, to correct the opinions of Chadwick, Cockcroft, and Joliot, who also had a candidate for the neutron mass. "I particularly want to make some rather extensive remarks on Cockcroft's report."[33]
Fig. 4.1
McMillan's apparatus for studying the absorption of gamma rays by various
materials. The rays scatter at right angles to the proton beam from the target
and enter through the mica window. McMillan, PR, 46 (1934), 868.
[31] Lawrence to Cockcroft, 2 Jun and 16 Sep 1933 (4/5); to Beams, 16 Sep 1933 (2/26); and to Swann, 28 Sep 1933 (17/3), quote.
[32] Lawrence to Cooksey, 5 Oct [1932] (4/19); Debye to Langevin, 8 Oct 1932 and 16 Sep 1933 (Langevin P, "Solvay 1933").
[33] Lawrence to Langevin, 4 Oct 1933 (Langevin P, 75). Each participant was asked to state the points in others' reports on which he wished to speak; Bethe to Langevin, 10 Oct 1933 (Langevin P, "Solvay 1933").
Cockcroft ended his report with an unenthusiastic review of Berkeley work. He accepted the alpha particles from the bombardment of lithium and presented the data about the 18-cm protons, but declined to entertain the hypothesis of disintegration. "It is rather superfluous to discuss further the nature of the transformations with proton emission until we have more experimental information." And how to get the information? From improved Cockcroft-Walton machines. The weak current the cyclotron brought to the target, one-thousandth the flux from the "direct" Cambridge method, might well wash out the advantage of the greater efficacy of its faster particles. "Our present information does not suffice for prediction."[34]
Lawrence's extensive comments centered on the cyclotron method and its latest achievements—hydrogen-molecule ions of over 5 MeV, deuterons of 3.6 MeV, a promise of protons at 3.5 MeV, evidence of the disintegration of heavy hydrogen in the fields of target nuclei, and numbers that made the neutron's mass unity. These last remarks made Lawrence himself the object of a bombardment. Heisenberg observed that if disintegration occurred in the electric field of a nucleus, the yield should decline for heavy targets since the deuteron's penetration, and hence the rate of change of force on it, must decrease with increasing atomic number (Z ); for (very) high Z the field would appear to the deuteron to change adiabatically and produce no disintegration at all. That being the case, added Bohr, we might suppose that the deuteron splits after entering a nucleus; but then the speed of the ejected proton should increase with atomic number, like the nuclear Coulomb field, contrary to Lawrence's results.[35]
Then came the experimentalists. Rutherford said that he had found no neutrons from lithium under deuteron bombardment. Chadwick reaffirmed the value of the neutron mass at between 1.0067, which he deduced from the hypothetical reaction B11 (a ,n)N14 , and 1.0072, which he had deduced from Li7 (a ,n)B10 . Joliot and Curie came forward with a neutron still heavier than Chadwick's. Their careful examination of decay products of
[34] Cockcroft, Solvay, 1933, 50–5; cf. Oliphant, PT, 19:9 (1966), in Weart and Phillips, History , 181.
[35] Solvay, 1933, 71, 72.
alpha-bombardment of boron and other light elements had disclosed quantities of positive electrons along with neutrons. They supposed that these particles came away simultaneously, according to the reaction B10 (a ,ne+ )C13 , and constituted when together the familiar proton. In place, therefore, of Chadwick's initial conception, that n = p + e– , they now proposed p = n + e+ . So much, and much more, was tied up in the question of the neutron mass. Calculations based on the transmutation of B10 made mn = 1.012. This big mass had the advantage of accounting for the stability of the hydrogen atom, since it prevented the spontaneous union of its proton and electron into a neutron; but it made the decay of the neutron into a proton, electron, and neutrino energetically possible.[36] The only difficulty that Joliot and Curie saw with their fat neutron was the conflicting experience in Berkeley. They were prepared to compromise: "It is not impossible that it will be necessary to suppose the existence of neutrons with different masses," theirs being the elementary one and Lawrence's a condensed combination of the elementary with an electron-positron pair.[37]
Lawrence responded to these challenges by invoking suppositious gamma rays, whose inclusion in the energy balance would lower the neutron mass. Chadwick denied the gammas and insisted, against Joliot and Curie, that the neutrons came from the more plentiful isotope B11 , with the mass he had assigned them. After this exchange the theorists could only feign hypotheses and await the outcome of the squabble.[38] As perhaps no one outside France expected, victory eventually fell to Joliot and Curie.
[36] Solvay, 1933, 77, 101–2, 155–6; Curie and Joliot, CR, 197 (1933), 237 (17 Jul 1933), in Oeuvres , 417–8; Langevin, "Rapport sur les titres et travaux de M. Frédéric Joliot" (Langevin P, 73/2); Pauli to Joliot, 1 Feb 1934, in Pauli, Briefwechsel, 2 , 271.
[37] Joliot, Solvay, 1933, 156. This was to recur to the hypothesis of A. von Grosse, PR, 43 (1933), 143, who supposed neutrons of various masses to explain the energy spctrum in beta decay. Cf. Pauli to Heisenberg, 14 Jul and 30 Sep 1933, in Pauli, Briefwechsel, 2 , 185, 216.
[38] Solvay, 1933, 165–9; Pauli to Heisenberg, 17 Apr 1934, in Pauli, Briefwechsel, 2 , 316. Chadwick had wanted to talk about the mass and nature of the neutron (letter to Langevin, 13 Oct 1933, Langevin P), but, apparently, not to listen.
On his way back to Berkeley, Lawrence stuck his head into the Cambridge lions' den. Chadwick bared his claws, to such effect that his behavior needed explanation. It was found in the consideration that he had been the effective director of the Cavendish for some time and was too overworked to observe the niceties of philosophical combat. With the other lions, especially Cockcroft and Rutherford, who licked his chops over Berkeley's "broth of a boy," Lawrence got along well. They merely roared in a friendly way against the hypothesis of deuteron disintegration and pointed their paws at the possibility that Lawrence had contaminated his targets and tank.[39]
Back at home Lawrence mobilized Lewis and switched Livingston and Henderson from trying to withdraw a beam from the cyclotron to clearing up the enigma of the 18-cm protons. Livingston arranged a target holder that would make possible bombardment of many samples in succession to test possible contamination.[40] Working night and day through the Thanksgiving holiday, Lawrence and his group found the yield of protons from deuterons to be unaffected by their efforts to clean up their targets. "Perhaps before long the evidence will be such as to convince the most skeptical, including those at Caltech and even Chadwick."[41]
The Caltech team, Crane and Lauritsen, suggested several possible complications in the analysis of Berkeley's experiments (for example, that the neutron found in deuteron bombardment of lithium might come from Li7 (p)2a followed by Li7 (a ,n)B10 ), and could find no trace of the 18-cm protons.[42] Then Tuve's group, which had the only machine then capable of checking Berkeley's results above a million volts, entered the picture. Lawrence had visited the Carnegie Institution on the way back from Brussels. "I persuaded Tuve to investigate the origin of the 18 cm protons and
[39] Edward Pollard to Lawrence, 6 Dec 1933, and reply, 20 Dec 1933 (14/30); Lawrence to Cockcroft, 20 Nov 1933 (5/4); Cockburn and Ellyard, Oliphant , 74. Rutherford to Lewis, 30 Oct 1933 (Lewis P): "He is a broth of a boy, and has the enthusiasm which I remember from my own youth."
[40] Lawrence to Cockcroft (5/4), to Cooksey (4/19), and to Darrow (6/9), all 20 Nov 1933.
[41] Lawrence to Gamow (7/25), to Poillon (15/16A), and to Haupt (9/2), quote, all 4 Dec 1933.
[42] Crane and Lauritsen, PR, 44 (Nov 1933), 783–4 (letter of 14 Oct).
the hypothesis of the disintegration of the deuteron right away," Lawrence wrote Cockcroft. "I want to get the matter cleared up as soon as possible and it will be a great help if Tuve, with his independent set up, will investigate the problem."[43]
The experiments went forward everywhere at an American pace. Lewis prepared two samples of calcium hydroxide, one with ordinary and the other with heavy hydrogen. Under bombardment by 3 MeV deuterons the ordinary target showed nothing extraordinary, whereas the heavy target yielded a cornucopia of 18-cm protons. What could be clearer? The bombarding protons broke up the bound deuterons. Lawrence dispatched this "unambiguous proof of d[e]uton disintegration" to the Cavendish; "It would seem now that even Chadwick will agree."[44] The same message went to the East Coast, to Pollard at Yale ("these recent observations definitely rule out the possibility of impurities") and Beams at Virginia ("the deuton is energetically unstable and disintegrates into a proton and a neutron"); to all other physicists through the Physical Review ; and, of course, to the Research Corporation. "We have proved beyond any reasonable doubt that the deuton explodes when struck hard enough. . . . This first definite case of an atom that itself explodes when properly struck is of great interest, not only as a possible source of atomic energy, but especially because it is not understandable on contemporaneous theories. . . . [It] promises to be a keystone for a new theoretical structure."[45] The flaw in the hydroxide experiment, which we shall reveal in a moment, was no more subtle than the hint about cheap energy. So far did hope, ambition, impatience, and a need for benefactors drive Lawrence from the objectivity he would have claimed as the first virtue of the scientist.
When the Cambridge atom splitters returned from Brussels, they had a fountain of dilute heavy water and samples of almost
[43] Tuve to Lawrence, 30 Jan and 12 Sep 1933 (3/32); J.A. Fleming to Lewis, 9 May 1933 (Lewis P); Lawrence to Cockcroft, 20 Nov 1933 (5/4).
[44] Quotes from, resp., Lawrence to Walton, 20 Dec 1933 (18/1), and to Fowler, 28 Dec 1933 (7/12); Lawrence to Rutherford, 20 Dec 1933 (ER); Lewis, Livingston, Henderson, and Lawrence, PR, 45 (1933), 497.
[45] Lawrence to Pollard, 20 Dec 1933 (14/30), to Beams, 28 Dec 1933 (2/26), and to Poillon, draft enclosing report for the year 1933, 15 Dec 1933 (15/16A); Lewis, Livingston, Henderson, and Lawrence, PR, 45 (1934), 242–4 (rec'd 3 Jan).
pure deuterium created in their absence by the finally successful, and consequently now esteemed, Harteck. His achievement came in good time, since Lewis's still had run dry and he could supply nothing until just before the Solvay Congress.[46] Now, with sufficient stock to hand, Rutherford and a research student, A.E. Kempton, returned to the master's old game, let alpha particles from polonium plunge through deuterium gas (the inverse of deuterons on helium), and found no evidence of fast disintegration protons.[47] Cockcroft and Walton sent deuterons against copper and gold and likewise detected no protons.
In the middle of December, Rutherford gave a speech at the Royal Society summarizing the latest evidence. He and Oliphant had at last found neutrons from deuterons on lithium, and Lauritsen neutrons from deuterons on beryllium; but whereas everyone associated these neutrons with nuclear transformations, Lawrence plumped for what Rutherford dismissed in a letter to Bohr as an "exothermal nucleus," and, together with Livingston, offered very shaky evidence that ruled out the reaction Li7 (d,n)2a as the source of the Cambridge neutrons.[48] Another Cavendish man, D.E. Lea, countered the hypothesis of the deuteron's instability by ascribing the hard gamma rays he observed from wax irradiated by neutrons to the spontaneous, endothermic formation of deuterons.[49]
[46] Harteck to Bonhoeffer, 21 and 24 Oct, 5 and 24 Nov, 2 Dec 1933 (Harteck P); cf. "Discussion on heavy hydrogen," PRS, A144 (1934), 27 (Rutherford), 10–1 (Harteck); Lewis to Rutherford, 5 Oct 1933 (Lewis P).
[47] Rutherford and Kempton, PRS, A143 (1934), 724–30, in Rutherford, CP, 3 , 377–83.
[48] Rutherford, Nature, 132 (23 Dec 1933), 955–6; Oliphant, Kinsey, and Rutherford, PRS, A141 (1933), 722–33, in Rutherford, CP, 3 , 358; Lawrence and Livingston, PR, 45 (1934), 220 (letter of 3 Jan). "Exothermal nucleus" comes from Rutherford to Bohr, 3 Jan 1934 (ER). Lawrence and Livingston's evidence: there appeared to be too many neutrons in comparison with the number of alpha particles to agree with the supposed reaction.
[49] Lea, Nature, 133 (6 Jan 1934), 24. Lea's observations did concern deuteron synthesis, but not, as he thought, by direct action of the fast neutrons with the wax protons. Only after they have been slowed by collisions in the wax do the neutrons become very effective for making deuterons. Thus Lea, and Chadwick and Goldhaber, who questioned his results, missed the grand discovery of thermal neutrons later made by Fermi, who mentioned Lea's work. Goldhaber in Stuewer, Nuclear physics , 93–4, and in Hendry, Cambridge physics , 191–3; Fermi, CP, 1 , 757.
Lawrence sought protection behind his big gun. Your conclusions are "hardly justified," he told Cockcroft, since the Cavendish experiments had run at under 600 kV.[50] Beginning at 700 kV things became more interesting. Lauritsen had then recently found many neutrons from fast deuterons on beryllium, carbon, and even copper. That, Lawrence crowed to Livingston, amounted to unquestionable corroboration of their experiments. "Chadwick will have to come down off his high horse now." The word at the Laboratory was that Lawrence had "clinched his mass of the neutron—though the evidence [as Kurie rightly objected] is not as clear as I'd like to see." Why did Rutherford not find fast protons from alpha particles on deuterium gas?[51] Lawrence tried to convince the reigning theorist in the business, Gamow, whom he had met at the Solvay conference; Gamow found the fog of conflicting experimental findings too dense to penetrate and offered to "come to California and try to split nuclei by pure theory."[52]
The Fruit
The Cavendish experimenters instead resolved the matter by pure experiment. At the end of February 1934, Cockcroft reported that they had at last detected fast protons from deuteron bombardment of copper, iron, gold, and yttrium, at energies down to 200 kV. Also, and the eventual key to the problem: "Oliphant is getting queer results with H2 and H2 ." Oliphant and Harteck had made up targets like NH4 Cl containing heavy hydrogen. When bombarded with protons, the targets behaved as if constituted entirely of ordinary hydrogen. When shelled with deuterons, they gave off protons with a range of 14.3 cm, which the Cavendish researchers tacitly identified with Berkeley's 18-cm rays. And this irrespective of the deuterons' energy, as long as it exceeded 20 keV. At 100 keV the effect was too large for recording on the
[50] Cockcroft to Lawrence, 21 Dec 1933, and response to Cockcroft, 12 Jan 1934 (4/5).
[51] Livingston to Lawrence, 23 Jan 1934, and Lawrence to Livingston, 26 Jan 1934 (12/12); Kurie to Cooksey, 6 and 21 Mar 1934 (10/21).
[52] Lawrence to Gamow, 28 Dec 1933, and Gamow to Lawrence, 12 Jan 3[4] (7/25).
sensitive detector of the Oliphant-Rutherford apparatus, so great in fact that Oliphant and Rutherford at first ascribed it to a burst of x rays. Analysis of range and energy, confirmed by cloud chamber pictures, showed that the protons arose from the reaction d+d ® H3 +p. They also found the neutrons that Lawrence had advertised. Rutherford discerned their origin in the competitive reaction, d+d ® He3 n.[53] Oliphant broke the news gently. "We suggest very tentatively that your results may be explained as due to the bombardment of films of D and of D compounds. . . . I hope these results are of interest to you."[54]
The puzzle Lawrence started had therefore the simple solution of contaminated apparatus: deuterons stuck in the walls of the cyclotron chamber and in the targets gave the plentiful d-d reaction when bombarded by deuterons; the fast protons, apparently from everything, which had prompted the disintegration hypothesis, did in fact come from deuterons, not from the target, but in consequence of fusion, not disintegration. The accelerator men at Caltech and Carnegie also offered plentiful evidence that Berkeley's results came from synthesis of deuterons with pervasive contaminants. Lauritsen and Crane showed that the fast protons from deuterons on carbon came from C12 (d,p)C13 , an observation extended by Cockcroft and Walton to explain, as a consequence of oil films on metals, why deuterons appeared to knock protons from copper and tungsten. Tuve and Hafstad repeated all the Berkeley experiments with carefully controlled beams on immaculate targets and found that the Berkeley experimenters had got nothing right. With a chamber contaminated with deuterons, however, they had no trouble recovering Berkeley errors.[55] The demonstration with the hydroxide targets, which Lawrence had thought so convincing, also failed through ubiquitous deuterons.
[53] Cockcroft to Lawrence, 28 Feb 1934 (4/5); Oliphant to G.P. Thomson, 9 Mar 1934 (GPT, D/1), on Dee's confirmation with the cloud chamber; Dee, Nature, 133 (14 Apr 1934), 564; Oliphant, Harteck, and Rutherford, Nature, 133 (17 Mar 1934), 413, in Rutherford, CP, 3 , 364–5, expanded in PRS, A144 (1934), 692–703, in CP, 3 , 386–96; Cockburn and Ellyard, Oliphant , 53–5.
[54] Oliphant to Lawrence, 12 Mar 1934 (10/16).
[55] Lauritsen and Crane, PR, 45 (1934), 345–6 (letter of 15 Feb); Cockcroft and Walton, PRS, A144 (1934), 704–9, 717–9; Tuve and Hafstad, PR, 45 (1934), 651–3 (letter of 14 Apr).
As Lauritsen and Oliphant independently explained it to Lawrence: the proton beam playing on Ca(DH)2 liberated deuterons by collision or exchange; these deuterons then joined the bombardment and produced the 18-cm protons by d-d synthesis.[56]
The hunters horsed to catch the hare let loose from Berkeley were formidable: the Cavendish from the top down and the experienced teams and big machines at two of the best-endowed physics research institutes in the United States. Resolution of the conflicting results required very careful experiment in previously unexplored regions of multiple, competing nuclear reactions; and it required, and abetted, discovery of the occult d-d fusion process and the isobars of mass three. That detection and analysis of deuterium fusion was not so easy as its plentiful yield might suggest appears from the struggles of Otto Frisch, who proposed to Bohr that they build a big source of neutrons utilizing the d-d reaction. He had a machine available in December 1934. As late as the following March he had not detected a single neutron, although he commanded 60 kV, far above the threshold reported by Oliphant, Harteck, and Rutherford. He did succeed eventually, as did G.P. Thomson, who also had trouble reproducing the Cambridge results.[57]
In accordance with the social expectations of science, Lawrence confessed himself chagrined at the "stupidity" of his error, and Cockcroft softened the blow with the falsehood that "for a long time Rutherford and Chadwick were nearly convinced that you were right" and the truth that the experiments of Lawrence, Lewis, and Livingston had brought "an important enlargement of the field of nuclear research."[58] Lawrence diagnosed his stupidity as a consequence of the great productivity of the cyclotron, which
[56] Lawrence to Cockcroft, 14 Mar 1934 (5/4), and Lewis, Livingston, Henderson, and Lawrence, PR, 45 (1934), 497 (letter of 15 Mar), re Lauritsen's suggestion; Oliphant to Lawrence, 12 Mar 1934, and Lawrence to Oliphant, 5 June 1934 (10/16). Kurie to Cooksey, 16 Mar 1934 (10/21): "Lauritsen has thrown the final monkey wrench in the disintegration hypothesis."
[57] Frisch to Meitner, 25 Mar, to Bohr, 29 Jun, and to Rausch von Traubenberg, 28 Dec 1934; to Meitner and to his father, 10 Mar, and to Jacobsen, 5 Apr 1935; and to Meitner, 27 Oct 1937 (Frisch P); Thomson to Oliphant, 5 Mar 1935 (GPT).
[58] Quotes from, resp., Lawrence to Cockcroft, 14 Mar 1934, and Cockcroft to Lawrence [1934] (4/5), and Cockcroft and Walton, PRS, A144 (1934), 704.
discouraged "methodical, quantitative, measurements;" a healthy point of view that enabled him soon to dismiss the experiments that had prompted his error as "of the character of a preliminary survey."[59] He had persisted in his errors, however, in the face of warnings from many sides of the likelihood of contamination.[60]
This flouting of good advice and good procedure irritated some of Lawrence's closest associates. Tuve wrote Cockcroft: "From our own experiments we feel that the important issue is rather one of judgment and point of view rather than of the errors in technique which can give rise to such a situation." Lawrence had botched everything so badly that the corrective, as Tuve wrote in an internal report, "will be a difficult thing to present in public." Kurie wrote Cooksey: "The Englishmen are doing what seem at this distance to be clean experiments, but Ernest and Malcolm [Henderson] are too excited to go slowly." These expressions of disappointment appear to reflect a worry that Lawrence's way of doing science and his rising celebrity in the United States might compromise American physics just as it was assuming world leadership. Cockcroft agreed that Lawrence's style did not suit nuclear physics. "There is a real danger of the subject getting into a mess, and I feel that the only thing to do is to delay publication until we are reasonably sure."[61]
Tuve did not hide his irritation from Lawrence. Their different institutional settings and personal ambitions had become more important in determining their scientific ethos than their similar backgrounds and almost identical scientific interests. In the spring of 1934 Tuve advised Lawrence to withdraw his faulty claims formally, a step not only ethically sound but also useful to Tuve, who would be spared the obligation of a public exposé. Lawrence replied petulantly. In the summer of 1934 Tuve carried the attack
[59] Quotes from, resp., Lawrence to Rutherford, 10 May 1934 (ER), and to Beams, 13 Apr 1934 (2/26); Lawrence, PR, 47 (1935), 17; Oliphant, PT, 19:9 (1966), in Weart and Phillips, History , 182–4.
[60] E.g., in letters from Boyce, 23 Jan 1933 (3/8), Cockcroft, 30 Mar 1933 (5/4), and Fleming, 10 May 1933 (3/32).
[61] Tuve to Cockcroft, 18 Apr 1934, and Tuve, "Memorandum," 3 Apr 1934 (MAT, 5); Cockcroft to Tuve, 30 Apr 1934 (CKFT, 20/80), the latter also in Hartcup and Allibone, Cockcroft , 65; Kurie to Cooksey, 4 Mar 1934 (10/21). Cf. Beams to Lawrence, 7 Apr 1934 (2/26), and Tuve, Hafstad, and Dahl, PR, 48 (1935), 316, in Livingston, Development , 29.
to Berkeley, to a meeting of the American Physical Society. Either the hospitality or the prevailing genius of the place caused the secretaries who reported the discussions to nod: they attributed to Tuve the irenic solution that his and Lawrence's results could be brought into harmony by considering the different apparatus and energy with which they worked. Not a mention of contamination or haste. Tuve could not allow that to pass, since it implied that not Lawrence, but he, had been wrong. He therefore wrote to Science , which had published the irenic report, to point out that the Berkeley group had abandoned their major claims and withdrawn many of their results, "which have been sought for but not verified," and which, by confusing the subject, had "delayed some-what the examination of these questions."[62]
To recover his standing, Lawrence thought to make a few "precise and trustworthy measurements." The deuteron business brought home forcefully, he told Cockcroft, that most of the Laboratory's fast data had no value. Accordingly, Lawrence made an elaborate study of the excitation and decay products of the useful isotope radiosodium, a novelty whose discovery will occupy us presently. Up to the maximum energies he used (1.9 MeV), deuterons seemed to induce transformations in sodium in the amounts predicted by Gamow's theory. But continuation of the experiments to aluminum and to higher energies in collaboration with a new recruit to the Laboratory, a postdoc from Princeton named Edwin McMillan, who knew how to work exactly, showed excitation larger than Gamow allowed. The departure of their measurements from a theory that worked well for protons and alpha particles interested Oppenheimer and his student Melba Phillips, who had just finished failing to explain the curious rise of absorption beyond the series limit in Lawrence's old reliable measurements on the photoeffect.[63]
When a fast deuteron enters a heavy atom, Coulomb repulsion slows its proton and so increases the relative velocity of its constituents. Under these circumstances, the nucleus may capture the
[62] Tuve to Lawrence, 17 Apr 1934, and response, 20 Apr 1934 (17/34); Tuve to Darrow, 28 Jul 1934, and to Cattell, 4 Aug 1934 (MAT, 13/"lab. letters"); Ward, Science, 80 (20 Jul 1934), 49, and Tuve, ibid., 17 Aug 1934, 161–2, quote.
[63] Lawrence to Cockcroft, 14 Mar 1934 (5/4), and to R.W. Ditchburn, 13 May 1933 (6/16).
neutron. The released proton then makes an appearance in the world. Encouraged by this attractive possibility, which occurred to several physicists, Lawrence, McMillan, and another new postdoc, Robert Thornton from McGill, pushed on to silicon and copper. Their results agreed exactly with the calculations of Oppenheimer and Phillips for a binding energy of the deuteron—about 2 MeV—that agreed very well with values obtained from mass-energy balances in nuclear transformations as measured by others.[64] Here was a point for the underdog. "I am amazed at the agreement," Lawrence wrote Rutherford. And also incurable: with the new mechanism, deuterons with available energies might provoke nuclear reactions "much further up the periodic table than one could ever have hoped for."[65]
There was another point for Lawrence's side of the story. Maurice Goldhaber, an émigré research student at the Cavendish, suggested to Chadwick an idea for an experiment that he had brought from Berlin: the photodisintegration of the deuteron. Goldhaber recalled that Chadwick showed little interest in the matter until told that if successful it would give data from which the neutron's mass could be deduced.[66] Telltale protons announced the disintegration of deuterons in heavy water irradiated by the hardest photons then available, the gamma rays of 2.6 MeV from ThC", of which the Cavendish had a good supply. With a preliminary determination of the kinetic energy of the proton, Chadwick and Goldhaber had a value of the neutron's mass without making assumptions about the mass of complex nuclei:
mn = md – mp + Eg – 2Ep» 1.0081.
Their value, almost midway between Chadwick's and the Joliots', is close to the modern one (1.0085). Since the mass of the proton was known to be 1.0078, it followed that Chadwick's neutron was radioactive. Its decay was first observed in 1948.[67] The discovery
[64] Oppenheimer and Phillips, PR, 48 (1935), 500–2. Cf. Pollard to Lawrence, 6 Dec 1933 (14/30); Oppenheimer to Bethe, "27 Nov," and Fleming to Bethe, 7 June 1935 (HAB, 3).
[65] Lawrence, McMillan, and Thornton, PR, 48 (1935), 494; Lawrence to Rutherford, 17 Apr 1935 (15/34), quote; Lawrence, Ohio jl. sci., 35 (1935), 404.
[66] Goldhaber in Stuewer, Nuclear physics , 84–8, summarized in Goldhaber in Hendry, Cambridge physics , 190–1; Bethe to F.W. Loomis, 5 May 1938 (HAB, 3).
[67] Chadwick and Goldhaber, Nature, 134 (1934), 237, disconfirming Lauritsenand Crane, PR, 45 (1934), 550–2 (letter of 24 Mar), whose careful study of Li (d,n)2a had recovered Chadwick's earlier value (mn = 1.0068), and Curie and Joliot, Nature, 133 (12 May 1934), 721, whose review of (a ne ) reactions had confirmed their own value (mn = 1.010). Cf. Ladenburg, PR, 45 (1 Feb 1934), 224–5, and PR, 45 (1 Apr 1934), 495; Chadwick, Feather, and Bretscher, PRS, A163 (1937), 366–75, giving mn = 1.0090. Chadwick and Goldhaber, PRS, A151 (1935), 479–93, predicted the decay of the neutron; the first observations took place in reactor laboratories after World War II. Feld in Segrè, Exp. nucl. phys., 2 (1953), 217–8.
of the photodisintegration of the deuteron gave rise to a new branch of nuclear investigation, to which the Berkeley Laboratory, which preferred to shoot particles it could accelerate, contributed very little. For a time the (g ,n) reaction appeared to be a promising source of neutrons.[68]
And finally, a point to be dilated later, the discovery of the d-d reaction, although instigated by cyclotron experiments, did not much recommend cyclotrons to nuclear physicists. Certainly it helped confirm the opinion of Rutherford, who had outplayed Lawrence with beams ten or even a hundred times less energetic than Berkeley's, that the Cavendish had no need for a cyclotron. More generally, it made possible a cheap substitute for the energetic neutron beams that, for a brief time, had been the peculiar preserve of large accelerators. It was now only necessary to have a supply of heavy water and a Cockcroft-Walton or Van de Graaff that could develop 100 or 200 kV, and one had a copious current of fast neutrons almost equal in energy courtesy of the peculiar sociability of deuterons.[69]
2—
A Fruitful Business
The episode with the deuterons exposed weaknesses in the work of the Radiation Laboratory that were only partly corrected before its mobilization in World War II. The pressure for quick results to encourage financial backers continued, with consequent hype and hurry. Errors plagued output: as Lawrence anticipated in
[68] Among the first to follow up the demonstration by Chadwick and Goldhaber were Szilard and the chemist T. Chalmers, who recommended radium gamma rays on beryllium. Szilard and Chalmers, Nature, 134 (29 Sep 1934), 494–5, in Szilard, CW, 1 , 145–6.
[69] Cf. Feld in Segrè, Exp. nucl. phys., 2 (1953), 380–4.
answering Cooksey's condolences over the death of the disintegration hypothesis, the Laboratory's mission and method guaranteed mistakes. "I have gotten over feeling badly," he wrote. "We would be eternally miserable if our errors worried us too much because as we push forward we will make plenty more." He worried a bit about the consequences. As a palliative he proposed that meetings of theorists should always include leading experimentalists, who could certify the value of the data, "Theoretical physicists," said he, forgetting his own persistence in error, "so often are liable not to appreciate which experimental observations are trustworthy."[70]
The proportion of solid results did increase, however, owing partly to Lawrence's resolution, which he sometimes kept or imposed on others, to finish and write up one research project before rushing to another; and owing largely to the appearance in the Laboratory of experienced researchers, who could design and carry through their own projects. Their presence brought something of the Cavendish pattern to the Laboratory. Just as Cockcroft and Walton, Oliphant and Rutherford, Chadwick and Lea, Walton and Dee, formed the nuclei of small groups that tackled similar problems from different points of view, so now Franz Kurie and Edwin McMillan, National Research Fellows for 1933/34, helped break the Laboratory from exclusive preoccupation with Lawrence's programs for physical research and machine design.
Kurie spent the first six months of his fellowship year fashioning a fine cloud chamber on the principle of Tuve's instrument, which worked "well, damned well as a matter of fact."[71] McMillan spent the same time disenchanting himself with the research project that had brought him to the Physics Department and considering what he might do in the Laboratory. Both found rich research lines under the inspiration of a discovery that also established the purpose, and secured the financing, of the prewar Laboratory. This prepotent discovery was made in France by
[70] Lawrence to Cooksey, 12 Mar 1934 (4/19); to J.A. Fleming, 2 Jul 1935 (3/32), resp.
[71] Kurie to Cooksey, 4 Mar 1934 (10/21), quote; Kurie, RSI, 3 (1932), 655–7; Dahl, Hafstad, and Tuve, RSI, 4 (1933), 373.
Curie and Joliot as they ploddingly reexamined the reactions on which they had based their generous estimate of the mass of the neutron.
Induced Radioactivity
Lawrence was not the only one to suffer for his hypothesis in Brussels. Curie and Joliot ran into formidable opposition to their conception of (a ,ne+ ) reactions—the supposition that boron and aluminum transform under alpha bombardment, with the simultaneous emission of a neutron and a positive electron. In their view, (a ,ne+ ) paralleled (a ,p) and demonstrated that the proton consists of a neutron and a positron.[72] No one doubted the presence of the positive electrons: but, to avoid the heavy neutron and the complexity of the proton, most Solvay participants preferred to place the origin of the positron outside the bombarded nucleus. The subterfuge appeared to work for beryllium, which emits gamma rays as well as neutrons under alpha bombardment, for the gammas might later convert into pairs of positive and negative electrons. But as Curie and Joliot pointed out, this explanation could hardly hold for aluminum, which, according to their experiments, did not emit gamma rays under alpha bombardment and gave out very few (if any) negative electrons in comparison with its positives. Consequently they held to (a ,ne+ ), only to be shot down by Lise Meitner, who had found no neutrons from alpha irradiation of aluminum.[73] Her faulty observation, which she later retracted, was to the Joliots what Chadwick's assurance was to Lawrence. They went back to their laboratory to prove their opponents wrong.
They thought that they could strengthen their argument by showing that neutrons and positrons appeared together and in equal numbers regardless of the energy of the incident alpha particles. Altering the incident energy required inserting absorbers between the polonium source and aluminum target; showing the associated production meant registering the positron on a Geiger counter and a conversion proton (from the neutron) in a cloud
[72] Curie and Joliot, JP, 4 (1933), in Oeuvres , 444–54, esp. 452–3.
[73] Solvay, 1933, 173–7.
chamber. All went as expected down to a certain energy, at which the conversion protons stopped, but not the positrons. Here Joliot confirmed the suspicion that Thibaud had expressed at the time of the Solvay Congress, that some radioactive bodies can emit positive electrons.[74] Joliot next tried the experiment with alpha particles of full energy. After the irradiation he removed the polonium source altogether. Still the positrons appeared for their allotted three minutes.
Joliot had made the aluminum radioactive by hitting it with alpha particles. He had discovered a two-step process, an (a ,n) reaction resulting in the creation of a new, unstable isotope of phosphorus followed by a positron decay to a stable isotope of silicon. The two-step achieved the same end as the single, straightforward, old-fashioned reaction (a ,p) would have procured. The intermediate product in the two-step brought not only confirmation of the Parisian heavy neutron, but something much more important, and altogether new: artificially created radioactive substances, which could be identified chemically by the carrier technique developed to analyze the products of natural radioactive decay. With the help of his wife, Joliot demonstrated that the three-minute activity followed the chemistry of phosphorus and that the fourteen-minute activity produced by (a ,n) on boron followed that of nitrogen. They brought a vial of one of their new creations to old Madame Curie, then dying of leukemia. Joliot described the scene. "I can still see her taking [it] between her fingers, burnt and scarred by radium. . . . This was without doubt the last great moment of satisfaction in her life."[75]
News of the discovery did not provoke much satisfaction when it reached Berkeley via Time and Nature . Lawrence, Livingston, and Henderson spent the weekend of February 24/25, 1934, repeating the experiments of Joliot and Curie in their own way, with deuterons from the cyclotron in place of alpha rays from polonium. "To our surprise we found that everything we bombar-
[74] Breit to Tuve, 9 Oct 1933 (MAT, 12/"spec. letters").
[75] Joliot, quoted in Goldsmith, Joliot-Curie , 57; Curie and Joliot, CR, 198 (15 and 29 Jan 1934), 254, 559, in Oeuvres , 515–9, and Nature, 133 (1934), 201, 721 (reaffirming the neutron mass), in Oeuvres , 520–1. Cf. Amaldi, Phys. rep., 111 (1984), 109.
ded . . . is radioactive." And also to their chagrin. "We have had these radioactive substances in our midst now for more than half a year. We have been kicking ourselves that we haven't had the sense to notice that the radiations given off do not stop immediately after turning off the bombarding beam."[76] It was not that the effect hid near the limit of detection: for aluminum it over-powered the Geiger counter. That made missing it—and the Nobel prize awarded to Joliot and Curie the following year—particularly galling. Later Lawrence's junior collaborators recalled what they remembered of their feelings. Thornton: "We looked pretty silly. We could have made the discovery at any time." Livingood: "We felt like kicking our butts."[77]
According to the standard apologies, the Laboratory missed the discovery because the same switch operated the cyclotron and the Geiger counter, and so turned off the means of detection with the initiating beam. It may be doubted that the equipment was so peculiarly wired. And even if it were, the fact that no accelerator laboratory thought to make substances radioactive remains to be explained. The Cavendish had looked for delayed activity in aluminum, among other elements, during the 1920s, with natural sources of alpha particles; they had found nothing, because, since neither the neutron nor the positron had yet been noticed, they had no idea what to look for, and sought to detect short-lived proton or alpha emitters with scintillation screens. As one frustrated investigator wrote, more truly than he knew, "It is very unfortunate that time did not permit of further experiments with a wide variety of elements and with devices for the detection of radiation of other kinds." Despite their larger sources and greater knowledge, accelerator builders did not reopen the matter.[78] It was not a question of labor-saving switches, but of labor-saving thinking. One expected either transmutation to known, stable species, or reduction to fundamental pieces of nuclei, but not the creation of brand-new radioelements.
[76] Lawrence to Beams (2/26), to J. Boyce (3/8), quote, both 27 Feb 1934; Kurie to Cooksey, 4 Mar 1934 (10/21).
[77] Davis, Lawrence and Oppenheimer , 60.
[78] Shenston, Phil. mag., 43 (1922), 938–43, quote on 943; Blackett, PRS, A107 (1925), 357; Rutherford, Chadwick, and Ellis, Radiations , 312–3.
Joliot and Curie had raised the possibility that deuterons might create artificial activities in their announcement of their discovery in Nature . They gave C12 (d,n)N13 as an example. Four groups stood ready to follow up the suggestion: Cockcroft's, Tuve's, Lauritsen's, and Lawrence's. Cockcroft at first preferred his original projectile and made N13 by stuffing a proton into C12 . Later he and his associates confirmed (d,n) reactions on boron, carbon, and nitrogen at energies under 600 kV. Tuve did not interrupt his investigations of Berkeley's mistakes to follow up Joliot and Curie's suggestions. Lauritsen did. He sent preliminary results on (d,n) reactions for publication on the same day that Lawrence did.[79]
The difference in research objectives between Caltech and Berkeley deserves notice. Henderson, Livingston, and Lawrence examined fourteen elements, from lithium to calcium, under bombardment by 1.5 MeV protons and 3 MeV deuterons; they noticed signs of proton activation only in carbon and supposed the ubiquitous deuteron activation to arise via (d,e+g ) reactions. They gave few and only rough quantitative data, for example, a half-life of the boron activity of about two minutes. In an unpublished lecture, Lawrence conceded that none of the measurements could stand up to the "very significant experimental findings" of Crane and Lauritsen.[80] The Caltech group limited its initial studies to 0.9 MeV deuterons on beryllium, boron, and carbon, understood that the activities they created arose from (d,n) reactions, showed that the half-life of the activity made from carbon agreed with that of N13 as given by Joliot and Curie, had their colleagues Carl Anderson and Seth Neddermeyer confirm the existence of positrons in the decay of N13 by observations with the Caltech cloud chamber, showed that the gamma ray found at Berkeley probably came from electron-positron annihilation, and determined the half-life of the boron activity to be ten times as large as Berkeley made it. Where Lauritsen's group gave careful and reliable
[79] Joliot and Curie, Nature, 133 (1934), 201–2, and in Oeuvres , 521; Cockcroft, Gilbert, and Walton, Nature, 133 (1934), 328 (letter of 24 Feb), and PRS, A148 (1934), 225–40 (rec'd 26 Sep); Crane, Lauritsen, and Harper, Science, 79 (1934), 234–5 (letter of 27 Feb).
[80] Lawrence, "Outline of lecture on artificial radioactivity," n.d. (40/16).
information about a few features of the new terrain, Lawrence's characteristically bolted through an impressionistic survey.
The usual tendency in the Radiation Laboratory may have been strengthened in this case by the increasing difficulty in maintaining the hypothesis of deuteron disintegration and by Lawrence's desire to assimilate their earlier results to the great Parisian discovery and insinuate an anticipation of it. "Indeed, in the light of our recent experiments in which neutrons and protons were found to be emitted from many elements when bombarded with deutons, the possibility presented itself that in these nuclear reactions [!] new radioactive isotopes of many of the elements might be formed." So Henderson, Livingston, and Lawrence hinted in the Physical Review in 1934. Later and in private Lawrence may have claimed more. A representative of the Rockefeller Foundation recorded this remark: "[Lawrence] said that they had discovered artificial radioactivity before Joliot and Curie did, but wishing to be overly sure [!] of their results, did not publish and were taking time to repeat the work."[81]
Some Physics Fallout
Lewis had very probably been the instigator in the deuteron experiments.[82] An extremely clever man with a secure reputation as a chemist, he had little to lose by backing poor physics; a hasty man, guided by smell and inspiration, he was the worst sort of collaborator for Lawrence. Also, he had ideas about atomic and nuclear structure that differed in principle from those physicists entertained. He had sponsored a static atom, in which electrons stand at the vertices of a polyhedron centered on their nucleus, in competition to Bohr's dynamic-electron model. His colleague Wendell Latimer had extended the scheme to the nucleus, which he supposed to consist of as many alpha particles as possible joined together in equilateral pyramids. He had no place for neutrons; they come to life outside nuclei, by couplings of protons
[81] Henderson, Livingston, and Lawrence, PR, 45 (1934), 428–9 (letter of 27 Feb); Crane and Lauritsen, PR, 45 (1934), 430–2 (letter of 1 Mar); Amaldi, Phys. rep., 111 (1934), 115–18; Frank Blair Hansen, "Trip report," 3–13 Apr 1938 (RF, 1.1/205).
[82] Lawrence to Potter, Pierce, and Scheffler, 3 June 1935 (35/7).
and neutrons, couplings easily broken and reformed, in his opinion, so as to make hydrogen atoms, deuterons, mass-three helium, and so on.[83] It was a qualitative, tinker-toy world, in which one part—such as the strength of the stick holding the deuteron together—could be changed without doing violence to other parts. Even before the detection of heavy water, a student of Berkeley's nuclear family foresaw the exploitation of the cyclotron to check the chemists' physics. "It may be," he wrote, "the research by Professor Lawrence and Dr Livingston will offer means of proving or disproving it."[84]
Fowler was astonished at the eagerness and confidence with which Berkeley chemists did physics. "If they do any chemistry it's kept well out of sight."[85] The fiasco of his work with Lawrence did not discourage Lewis. In 1936 he offered an explanation of neutron scattering that was as disruptive to theory as exploding deuterons. Bethe reviewed the manuscript for the Physical Review : "I think it is an extremely instructive example of the dangers of purely qualitative arguments." Lewis's former confederates at the Laboratory would not follow him: "The effect [for which Lewis argued] is so feeble, and the instruments so barbaric (he doesn't want to hear about counters) that no one believes him here."[86] With this rejection, Lewis ceased to play a direct part in the work of the Laboratory.
Kurie did not allow himself to be drawn into the search for activities induced by deuterons. He took on instead the elucidation of the mechanism of neutron activation. He studied closely the forked tracks created in his cloud chamber during neutron irradiation of nitrogen. In contrast to Rutherford's prompt reaction N14 (a ,p)O17 , Kurie thought he saw the delayed reaction N14 (n)N15® B11 + a + Q , where Q designates the energy carried away in gamma rays and N15 is an "intermediate nucleus." Kurie reported this first piece of careful physics done with cyclotron
[83] Lewis, Valence ; Latimer, JACS, 53 (1931), 981–90, and JACS, 54 (1932), 2125–6; Kohler, HSPS, 3 (1971), 343–76.
[84] G.A Pettitt, California monthly, 27 (1931), 18–21.
[85] Fowler to Rutherford, 22 Mar [1933] (ER).
[86] Bethe to Buchta, 6 May 1936 (HAB, 3); Nahmias to Joliot, 28 Apr 1937 (JP, F25); Seaborg, Jl., 1 , 242; Lewis and Schutz, PR, 51 (15 June 1937), 1105.
beams and a good detector at a meeting of the American Physical Society in Berkeley in June 1934.[87] In the fall he gave a seminar on his work. "For the first time in my life [it] was not a recital of numbers but of ideas." Everyone seemed convinced, except Oppenheimer, who worried about the powerful gamma ray that, if the conservation laws held, must be emitted in the formation of the intermediate nucleus. "Robert says that the evidence is well explained by it but he 'wishes it were not so'"[88] Kurie published his hypothesis and measurements—the sort of paper Lawrence was "proud to have from the lab"—and it was not so. As Bethe laid down the law, in 1937: "This [intermediate excited nitrogen nucleus] has no justification either theoretically or experimentally, and has subsequently been discarded." Apparently Kurie had overinterpreted his tracks.[89] His work was not to end the Laboratory's stream of flawed physics.
At the same meeting in Berkeley of the American Physical Society at which Kurie spoke about delayed disintegrations, McMillan discussed preliminary results of his study of gamma rays excited by 1.15 MeV protons driven against fluorine. He had taken up the subject on the advice of Oppenheimer, who had two objects in mind. For one, the energy balance in nuclear reactions could not be struck without knowledge of the amount carried away by high-frequency radiation. For another, and of greater interest to Oppenheimer, gamma rays from some artificially induced reactions might well be more energetic than any from natural souces; if so, they would permit a check of the theory of pair production—the materialization of a gamma ray into a positron and an electron in the field of a nucleus—at higher energies than previously available. Oppenheimer and one of his students, Wendel Furry, had a calculation of pair production in such regions in hand.[90]
McMillan's experimental arrangement occupies figure 4.2. The Lauritsen electroscope consisted of a quartz fiber suspended from a wire and carrying on its free end a crosshair viewed against a
[87] Kurie, PR, 46 (1934), 324; Lawrence to Gamow, 19 May 1934 (7/25).
[88] Kurie to Cooksey, 18 and 27 Sep 1934 (10/21).
[89] Kurie, PR, 47 (1935), 98–105; Lawrence to Cooksey, 29 Dec 1934 (4/19); Livingston and Bethe, RMP, 9 (1937), 338, 339 (quote), 341.
[90] McMillan, PR, 46 (1934), 325, 868, 870.
scale in the microscope eyepiece. Foils of various metals allowed determination of the absorption of the gamma rays from the target as a function of atomic number Z , and thereby distinction of the portion owing to pair production (which increases as Z3 ) from contributions from the photoeffect and the Compton effect. McMillan took elaborate precautions not to be duped by contaminants. The best results came from fluorine, which produced fine energetic gamma rays, some 5.4 MeV, in the reaction F19 (p,a )O17 . Pair production by these rays agreed perfectly with the curvature of the tracks of the most energetic photoelectrons they produced in a cloud chamber at Caltech.[91] McMillan's were the first experimental results in nuclear physics obtained at the Laboratory and controlled by a quantitative theory that have stood up under bombardment from other investigators.
Tracer Business
While McMillan and Kurie went their independent ways and Lawrence's group continued firing deuterons, another capital discovery arrived from Europe. Fermi had reasoned that because they carry no electrical charge, neutrons should be able to gain admission to nuclei more readily than protons, deuterons, or alpha particles; and that they were the only way to activate nuclei heavier than phosphorus, where even Berkeley's deuterons could not penetrate.[92] A methodical man, Fermi began with hydrogen as a target, then lithium, and so on, at first with no luck. He was not discouraged before reaching fluorine, which released electrons when struck by the neutrons from his modest radon-beryllium source. That was on March 25, 1934. Fermi then mobilized his collaborators, Edoardo Amaldi, Franco Rasetti, and Emilio Segrè, who raced at California speeds to procure and bombard specimens of all the elements in the periodic table. By July they had reached uranium and detected no fewer than forty artificial isotopes of the
[91] McMillan, PR, 46 (1934), 871–2; Henderson, Livingston, and Lawrence, PR, 46 (1934), 38; Crane, Delsasso, Fowler, and Lauritsen, PR, 46 (1934), 531.
[92] Cooksey had suggested the effect Fermi sought the year before: "I suppose that the neutron in the H is the boy that when given an introduction in the company of a proton raises all this merry hell." Cooksey to Lawrence, 6 May 1933 (4/19).
Joliot-Curie type, but which decayed by emitting negative rather than positive electrons. Of particular interest, for reasons to appear, was Na24 , with a half-life of fifteen hours, which Fermi's group could create either from Al27 via (n,a ) or from Mg24 via (n,p).[93]
The discoveries of artificial radioactivity and of the capacity of neutrons to effect transformations beyond the reach of deuterons coalesced several disconnected ingredients of Lawrence's research, machine building, and fund-raising into an enduring whole. Even before Fermi's discovery, at the Solvay Congress of 1933, Lawrence had emphasized the importance of deuteron bombardment as a source of neutrons: with a current of only 0.01 µA the Laboratory had a source that appeared to be more powerful than any likely to be obtained from natural radioelements. One standard estimate, used by Fermi, gave 1,000 neutrons/sec as the output of one mCi of Rn-Be; another, used by Joliot and Curie, made the efficiency of nuclear reactions induced by alpha particles from natural radioactive sources about 10–6 or 10–7 ; hence 0.01 µA of deuterons, or 6.3·1010 particles/sec, would give rise to around 10,000 neutrons/sec, which would have required the radon from 10 grams of radium. This was an exaggeration. Later elaborate measurements by Amaldi and Fermi and by Amaldi, Hafstad, and Tuve raised the yield from a mCi of Rn-Be to 25,000 neutrons/sec and the conversion efficiency of 1 MeV deuterons on beryllium to 3·10–5 , whence 0.01 µA of million-volt deuterons would give as many neutrons as 70 mCi of Rn-Be. Fermi did his first experiments with 50 mCi of radon and at times used 700 mCi, which his supplier, G.C. Trabacchi, drew from a gram or so of radium at the Istituto di sanità pubblica in Rome.[94] Fermi's source of
[93] Segrè in Fermi, CP, 1 , 639–41; Fermi, Ric. sci., 5 (1934), 283, in CP, 1 , 645–6 (dated 25 Mar 1934), announcing (n,a ) reactions on F and Al; Nature, 133 (1934), 757, letter of 10 Apr, describing activities of two dozen elements; Fermi, Amaldi, D'Agostino, Rasetti, and Segrè, PRS, A146 (1934), 483–500, in CP, 1 , 732–47, esp. 746–7 (rec'd 25 Jul 1934). Amaldi, Phys. rep., 111 (1934), 130, considers the Rome group to have been "probably the first large physicists' team working successfully for about two years in a well organized way."
[94] Lawrence, intervention in Solvay, 1933, 68. Cf. Livingston, Henderson, and Lawrence, PR, 44 (1 Nov 1933), 782–3 (letter of 7 Oct); Fermi, Ric. sci., 5 (1934), 283, in CP, 1 , 645–6, and Nuovo cim., 11 (1934), in CP, 1 , 715; Amaldi, Fermi, Rasetti, and Segrè, Nuovo cim., 11 (1934), in CP, 1 , 725; Fermi, Amaldi,D'Agostino, Rasetti, and Segrè, PRS, A146 (1934), in CP, 1 , 733, 745; Amaldi and Fermi, Ric. sci., 7 (1936), in CP, 1 , 887, and PR, 50 (1936), in CP, 1 , 892, 937–8; Jaeckel, Zs. f. Phys., 91 (1934), 493; Paneth and Loleit, Nature, 136 (1935), 950; Amaldi, Hafstad, and Tuve, PR, 51 (1937), 896.
neutrons compared well with the cyclotron vintage 1933, but it could not compete in total output with later versions or with any other artificial source of a µA of deuterons above a million volts. Still, a natural source retained its usefulness in situations where constancy, reproducibility, and good geometry were especially important.
By April 1934 the Laboratory was busy following up Fermi's results, which greatly complicated inventorying nuclear reactions. "Because of the magnitude of the radioactivity induced by neutrons it was immediately apparent [Lawrence wrote Gamow] that we should study thoroughly the phenomena before trying to untangle other nuclear reactions produced by proton and deuteron bombardment." And there was another reason, as Lawrence wrote another interested party, with interests much different from Gamow's. "We are not unmindful [he told Poillon] of the possibility that we may find a substance in which the radioactivity may last for days instead of minutes or hours, in other words, a substance from which we could manufacture synthetic radium. The probability is not at all remote at the present time. I am very glad that we have a patent on the cyclotron."[95]
With the help of Henderson and Livingston, Lawrence got tremendous amounts of radioactive aluminum, copper, silver, and fluorine; owing to a new set of dees made wider at the center to accommodate a larger ion source, the cyclotron was putting out 0.7 µA of 3 MeV deuterons, which shook over 500,000,000 neutrons/sec from its beryllium target. The poor estimate then still accepted, 1 mCi of Rn-Be gives 1,000 neutrons, implied that the cyclotron had the value as a neutron source of half a kilogram of radium. (Rutherford then estimated the equivalent of Oliphant's machine as a tenth of a gram of radium).[96] When
[95] Lawrence to Poillon, 3 Mar 1934 (15/16A); cf. Lawrence to Kast, 13 Apr 1934 (12/32).
[96] Kurie to Cooksey, 4 Mar 1934 (10/21); Lawrence to Beams, 13 Apr 1934 (2/26), to Cooksey, 21 May 1934 (4/19), to Oliphant, 5 June 1934 (14/6); Livingston, Henderson, and Lawrence, NAS, Proc., 20 (1934), 470–5; Rutherford to Fermi, 20 June 1934, in Amaldi, Phys. rep., 111 (1984), 133.
Franco Rasetti arrived in Berkeley in September 1935 to investigate artificial neutron sources, the cyclotron could generate 9 µA of 3.5 MeV deuterons, and therewith ten billion neutrons per second. Rasetti was flabbergasted by the "enormous superiority" of an artificial method that yielded what, by the natural way and the standard conversion, would have required the radon from kilograms of radium. In Rome he had made a certain activity by placing a silver target on top of a 500 mCi source; in Berkeley he got the same amount of activity with the target fifteen feet from the cyclotron wall.[97]
Lawrence did not find it necessary to trouble his backers with the names of Joliot, Curie, and Fermi. In writing Poillon and Ludwig Kast, president of the Macy Foundation, he claimed, what was true, that he had been the first to induce radioactivity in a range of elements by deuteron bombardment and allowed them to infer that he had discovered artificial radioactivity. A similar invitation was offered in the news that "we have found that an analogous effect is produced by neutron rays." This skillful reporting brought $2,300 from the Macy Foundation and $5,000 from the Research Corporation for working up materials to make radioactive substances on a large scale.[98] In the summer of 1934, therefore, when the Depression had forced substantial cuts on the Physics Department and Sproul went begging for money for the University, Lawrence found himself in excellent financial shape to perfect his machine for a purpose that had not been dreamed of when he and his associates built it: the creation of new radioelements and their manufacture in quantities sufficient for biomedical research. Before biology, however, comes chemistry. The physicists and machine builders had to transmute themselves into part-time chemists to separate out the various activities created by their neutrons and deuterons and to judge the possible utility of their handiwork for biologists.[99]
[97] Rasetti, Viaggi, 3 (1936), 77–8 (Aug–Oct); Lawrence to Cockcroft, 12 Sep 1935 (4/5).
[98] Lawrence to Kast, 3 May 1934 (12/32), and to Poillon, 15 and 26 Mar 1934 (15/16A); Kast to Lawrence, 23 Apr and 5 June 1934 (13/32); Lawrence to Sproul, 20 Feb 1936 (20/19).
[99] Lawrence to Beams, 17 Sep 1934 (2/26); Pettitt, Twenty-eight years , 40–1, 63.
It remained to find something useful. The plum fell to Lawrence, in September 1934, just after he had lamented to Beams that "the nuclear reactions we have been studying are not particularly novel."[100] Perhaps ignorant that Fermi's group had made the active isotope of sodium, Na24 , by (n,a ) on aluminum and (n,p) on magnesium, Lawrence did the same, by deuteron bombardment of table salt. But whereas Fermi's group had merely reported the existence and half-life of the product, Lawrence, from his special perspective, immediately emphasized the properties that might make it useful for "the biological field." These included its convenient half-life (fifteen hours), its nontoxic chemical character, and the energetic gamma ray that accompanied its disintegration.[101] And, what Lawrence did not make explicit, the fact that the valuable Na24 was isotopic with ordinary sodium made it unnecessary to remove the radioactive atoms from their parent for application. The target and the converted atoms could be administered together.
The essential property of radiosodium for biological research and medical application was the gamma ray emitted by the excited magnesium atoms produced by the decay of Na24 . Lawrence established that the reactions at play are Na23 (d,p)Na24 , Na24® Mg24 * + e– , Mg24 * ® Mg24 + g ; and he estimated that the gamma ray had an energy of over 5 MeV. He was pleased with this result, which made the gamma ray from radiosodium more than three times as hard as the hardest ray from radium, and which showed that he was still capable of solid scientific work. But not precise work. As one of his students, Jackson Laslett, soon showed, his determination of the gamma ray energy erred by excess by about 30 percent.[102] (In fact, the energy of the most energetic gamma ray from Na24 is under 3 MeV.) A gamma ray of 3 MeV was none the less a very useful item in physical research; members of the Laboratory used it to study pair production and the photodisintegration of the deuteron. It also retained its
[100] Birge, History, 4 , xi, 12; Lawrence to Beams, 17 Sep 1934 (2/26).
[101] Kurie to Cooksey, 27 Sep 1934 (10/21); Lawrence to Livingston, 1 Oct 1934, and answer of 12 Oct (12/12), mentioning Fermi's work; Lawrence, "Notebook," 27 Sep 1934 (40/14), and PR, 46 (1934), 746.
[102] Lawrence, PR, 47 (1935), 25; Lawrence to Cockcroft, 12 Feb 1935 (4/5).
promise for the health sciences. Lawrence's hopes could easily sustain a reduction of 30 percent. He wrote in December 1934: "We have succeeded in producing radioactive substances that have properties superior to those of radium for the treatment of cancer, and probably before long we shall make available to our medical colleagues useful quantities of radiosodium."[103]
The rate of production rose quickly. Within two months of first production, more than a mCi of radiosodium had been made and improvements in manufacture were under way that would increase the rate a hundredfold. Two years later Lawrence could make 200 mCi a day, with a current of only 1 µA, and he looked forward to multiplying the yield a thousandfold. With 20 µA, a day's product of radiosodium emitted the equivalent in gamma radiation of 100 mg of radium.[104] These production levels made an impact. Fermi supposed that Lawrence had slipped by a factor of a thousand and had meant to announce a µCi; Lawrence silenced his doubts by sending him a letter containing a mCi of Na24 . Wilhelm Palmaer, president of the Nobel Committee on Chemistry, highlighted the promise of a cornucopia of radiosodium during the ceremonies in which Urey and Joliot and Curie received their prizes. In another happy omen, the Rockefeller Foundation, the greatest patron of biophysics in the 1930s and eventually Lawrence's most generous private benefactor, advertised radiosodium as the exemplar of the cost-effective service to mankind it liked to support.[105]
The salt to make the equivalent of a gram of radium cost less than a penny (in fact much less, since the Myles Salt Company of Louisiana donated crystals of rock salt), and the power for the eight-hour exposure in the cyclotron less than $2. Putting the same point a different way, Science Service headlined its report of the meeting of the American Physical Society of December 1936, at which the Laboratory's Paul Aebersold announced Berkeley
[103] Lawrence to Cooksey, 4 Nov 1934 (4/19), on pair production; Nahmias to Joliot, 27 June 1937 (JP, F25), on photodisintegration; Lawrence to E.B. Reeves, Commonwealth Fund, 7 Dec 1934 (10/18).
[104] Lawrence to Akeley, 14 Nov 1934 (1/12), and to Cooksey, 12 Sep 1936 (4/5); Lawrence and Cooksey, PR, 50 (1936), 1140.
[105] Segrè, Ann. rev. nucl. sci., 31 (1981), 7; Palmaer in Prix Nobel en 1935 , 38, and Nobel lectures, chemistry, 2 , 337; Rockefeller Foundation, "Trustees confidential bulletin," Dec 1937, "Atom smashing and the life sciences" (RF).
production levels, "Machines of science produce radiation equal to $5,000,000 worth of radium." The calculation: the biological effect of the neutrons from deuterons shot at beryllium targets in the cyclotron equalled that of the gamma rays from 125 mg of radium. The reporter estimated the price of radium at $40 a mg and the cost of the cyclotron at under $100,000. "Thus as a radiation source the machine turned in a 50-to-one investment." And more: to get an equivalent neutron yield from a Rn-Be source would have required 10 kg of radium. That should answer "people who urge more practical scientific research and bemoan the apparently wasted ingenuity of those scientists who probe the hearts of atoms."[106]
Radium was passé. Lawrence advised his correspondents against investing in any of the stuff. Radioisotopes from the cyclotron, he said, would soon drive down the price of radium and supplant it in clinical use.[107] This advertisement attracted the attention of Bernard Lichtenberg, director of the Institute of Public Relations in New York. It did not seem good public relations to him, and he complained to Sproul. A mild reprimand went forward. "In explaining the work of the Radiation Laboratory," Sproul's assistant wrote Lawrence, "make sure the listeners realize that radium and radio-active salts are not the same."[108]
Lawrence reserved his more extravagant claims for presentation to his backers in private. He usually spoke modestly about the Radiation Laboratory in public, and allowed his audience to imagine what the future might hold. A lecture given at several colleges and universities under the sponsorship of the Sigma Xi in 1935 is representative. "I hesitate to express views [about the future]," Lawrence said. "I leave it to you to estimate the advantages for radiation therapy and biological research of radioactive substances having practically any desired chemical and physical properties." Two years later, in a second round of Sigma Xi lectures given at ten institutions from Virginia Polytechnic to Oregon State College during May 1937, he could point to the prospects for
[106] Myles Salt to Lawrence, 26 Nov 1934, and Lawrence's response, 4 Dec 1934 (11/24); Science service , 23 Dec 1936, re Aebersold's talk.
[107] E.g., Lawrence to G.M. Schrum, University of British Columbia, 7 Nov 1936 (2/29), and to Earl R. Crowder, M.D., Evanston, Ill., 4 May 1937 (5/5).
[108] G.A. Pettitt to Lawrence, 11 Mar 1937 (20/19).
biological research of machine-made radiophosphorus (P32 ) and radioiron (Fe59 ), the former found by Joliot and Curie in 1934, the latter a discovery of Berkeley cyclotroneers.[109] And he gave his audience a new basis for estimating the possibilities of his products. He had fresh samples of radiosodium airmailed to him for each performance. He called up volunteers, fed them radiosodium, and followed the course of the activity in their blood with a Geiger counter he carried with him. This "vaudeville," as he called it, held attention; no ear-witness could doubt "that we can make really strongly active substances."[110] Berkeley colleagues, including Oppenheimer, served as guinea pigs in local demonstrations, and would-be cyclotroneers elsewhere copied the show for their purposes. Lawrence was pleased to provide the main ingredient for these performances.[111]
Some indication of the impressions desired and the advertisments volunteered may be gathered from a radio interview in the spring of 1939 to which Lawrence brought his hot sodium. He passed a Geiger counter over it. Click, click. He asked his interviewer, Hale Sparks, to put the counter behind his back. Click, click. "You mean to say [Sparks cried] that the radiation is actually passing through my body now?" "Yes." Sparks: "Then the cyclotron has an unlimited future despite its great achievements of the past?" "Yes, indeed."[112]
[109] Lawrence, "Artificial radioactivity" (July 1935), 16 (40/17), quote, and Ohio jl. sci., 35 (1935), 405; letter to S.B. Arenson, 24 Sep 1937 (40/17); Newson, PR, 51 (1937), 624–7; Livingood and Seaborg, PR, 52 (1937), 135.
[110] Lawrence to McMillan, 5 and 11 May 1935, quotes (12/30); to M. Henderson, 3 May 1935 (9/6); and to Boyce, 18 Dec 1935 (3/8); McMillan, PT, 12:10 (1959), in Weart and Philipps, History , 263.
[111] E.g., Robley Evans to Cooksey, 25 June 1937, and to Lawrence, 17 Feb 1937, and Lawrence to Evans, 28 Jan and 23 Feb 1937 (7/8); S.J. Simmons to Lawrence, 8 and 20 Oct 1939 (16/24).
[112] Lawrence to H.A. Scullen, 21 Apr 1937 (40/17), and to Cooksey, 12 May 1937 (4/21); "University Explorer," "Adventures in science," 15 Apr 1939, 16 (40/15); George Volkoff to R. Cornog, 10 Mar 1941 (5/2).
3—
Business
The discovery of Na24 brought Lawrence into a new relationship with patents. In the case of the cyclotron, he had been persuaded to seek protection in order to block a possible monopoly by Raytheon or other firms. In the case of artificial radioelements, he participated in an effort to secure a monopoly position in a future radio-pharmaceutical industry. Here he and the Research Corporation were stymied by the difficulties in their case and by the practices of the U.S. Patent Office. A few European physicists did manage to secure patents on nuclear processes. The competitive environment influenced the direction of the work undertaken at the Laboratory, but did not restrict its openness to visitors or lessen its generosity to colleagues.
A Corner on the Market
Early in January 1932 the Research Corporation's lawyer, A.P. Knight, completed a draft application for a patent on the cyclotron, an instrument to produce high-speed ions by successive impulses "in a compact or relatively small apparatus." The ions, according to Knight, might be "utilized in any suitable manner, for example, for application to the disintegration or synthesis of atoms, or for general investigations of atomic structure, or for therapeutic investigations or applications." (Here the lawyer foresaw applications that Lawrence, still mired in machine design, probably had not; lawyers might be useful adjuncts to research teams.) Knight claimed injecting, accelerating, focusing, deflecting, and extracting the ions as patentable. The patent examiner rejected them all, as was the custom, enforced greater precision in language, and allowed the claims in September 1933.[113] The patent was granted in February 1934.
Shortly after the Research Corporation received Lawrence's assignment of rights in the cyclotron, its leaders visited Berkeley
[113] Draft application, 5 Jan 1932, filed with changes, 26 Jan, as "Method and apparatus for the acceleration of ions" (no. 589,033); correspondence between Knight and the Patent Office, 1932/33; Knight to Lawrence, 7 Jul 1932 and 15 May 1933; patent no. 1,948,384, 20 Feb 1934; all in 35/2. Cf. Vaughn, Patent system , 23.
to see the cyclotron in action and to encourage its inventor to stay alert to patentable material developed in the Laboratory. Lawrence became almost an agent for the Research Corporation. In September 1933 he proposed patenting the water-cooled anode at the tip of the coil in Sloan's x-ray machine; in 1934 he wanted to patent a cheap cloud chamber for lecture use; in 1938 he brought Beams' ultracentrifuge to the Research Corporation; in 1939/40 it was the turn of a new oscillator developed by Sloan and Lauriston Marshall, and in 1941 of an application to industrial radiography by a Berkeley engineer. Lawrence wrote the engineer, for whom he interceded with the Research Corporation, without a hint of the old physicist's ethos: "If you are going after any patents along this line, you have my blessing."[114]
The apparently fallow years in the preceding recital were, in fact, Lawrence's busiest and most vexatious time with patent affairs. A week after he had identified Na24 , he recommended his discovery to Knight and Poillon as "almost ideal for biological work," a novelty that "might ultimately supersede radium in usefulness." He added that by running fast enough, Knight could get an application to the Patent Office before news of Lawrence's type of radiosodium appeared in print. Poillon was ecstatic: the discovery fulfilled half the prophecy he had made six months earlier to the president of the Chemical Foundation, that Lawrence's results qualified him for a quick Nobel prize and that his further work would help bring about "the production of synthetic radium." Poillon ordered Knight to run.[115] Knight had the application in hand a week later; it emphasized that in Lawrence's process the radioactive material was chemically identical with the target and, moreover, that the chemical sodium does not harm the human body. The emphasis on the chemical identity of target and
[114] Poillon to Lawrence, 23 Feb 1932; Lawrence to Knight, 16 Sep 1933, both in 35/2; correspondence with Central Scientific Company and others, Jul–Aug 1934 (10/7), and Lawrence to Cooksey, 7 Jul 1934 (4/19); Lawrence to Poillon, 22 Jan 1938 (15/17A), and 27 Apr 1939 (15/18); Lawrence to John E. Dorn, Mechanical Engineering, Berkeley, 1 Mar 1941 (19/24). Infra, §10.2 for the Sloan-Marshall tube.
[115] Lawrence to Poillon, 29 Sep 1934 (35/7); Poillon to Knight, 1 Oct 1934, and to Buffum, 3 Apr 1934 (RC): "Both these statements are of such great importance that I forbid their being published lest it affect my standing as a Doctor."
product aimed to elude Fermi's patent on Na24 made by (n,a ) on aluminum. This was perfectly proper, since patent law made a clear distinction between process and product: controlling a product does not mean controlling all ways to make it, and vice versa.[116]
The patent examiner observed that the novelty claimed was production via (d,p) and that Crane and Lauritsen had priority of publication of results obtained in that manner. "There is no invention in utilizing the well known effects of deuteron bombardment upon any particular light metal," he held, and rejected all Lawrence's claims.[117] Although Knight insisted that the work of Crane and Lauritsen had nothing to do with Na24 , and although Lawrence declared that excitation by (d,p) was first observed at Berkeley, the examiner held firm. Meanwhile the Laboratory had found that (d,p) could make another important radiosubstance, P32 , which Lawrence urged Knight to try to patent together with the (d,p) process.[118] To this last proposal the examiner returned a crippling objection: the (d,p) process as described by Lawrence, Lewis, and Livingston in 1933, not the application of the process to sodium in 1934, should be the precedent; and, if Lawrence could not show that at that time he had the idea of activating the substances he bombarded, the game was up.[119] (A patent application could not then be filed on the strength of the paper of 1933, since filing had to occur within two years of publication.) This objection had at least this merit: it revealed how far the various parties were prepared to go to secure control of Lawrence's artificial radium.
The examiner held that since Na24 and P32 were produced by (d,p) in the experiments of Lawrence, Lewis, and Livington, they had been discovered then even though no one knew it. That construction of the legal mind nonplussed Lawrence. He proposed to
[116] Knight to Lawrence, 8 Oct 1934, enclosing application no. 748,085; Lawrence to Knight, 9 Oct 1934, all in 35/7; Vaughn, Patent system , 20.
[117] Knight to Lawrence, 21 Mar 1935 (35/7); Crane and Lauritsen, PR, 44 (1933), 783–4, 45 (1934), 226–7, 430–2, and Crane, Lauritsen, and Harper, Science, 79 (1934), 234–5.
[118] Lawrence to Knight, 2 and 16 Apr, 27 Aug 1935, and to Potter, Pierce, and Scheffler, 3 Jun 1935 (35/7); examiner's response, 21 June 1935 (35/7).
[119] Knight to Lawrence, 11 Sep 1935, and reply, 9 Oct 1935 (35/7).
Knight that they drop the attempt to patent radioelements. After all, he said, we have the cyclotron, "which I am sure will always be the apparatus that produces the radioactive substances." Lawrence thus reversed the estimate he had made during the patenting of the cyclotron. Then, while Poillon's Knight duelled with the examiner, Lawrence had doubted that the cyclotron had any commerical possibilities in the near term; and, when the duel concluded happily, he had questioned whether the Research Corporation should go to the additional expense of securing a Canadian patent. "The patent application on the cyclotron is very much of a gamble. It may never be of great worth," he had written Knight. "And yet," he added, "developments may come which would make it of tremendous value."[120] The developments that revalued the cyclotron were, of course, the discoveries of artificial radioactivity and neutron excitation.
Poillon declined to hide behind the cyclotron. He appealed to Lawrence's patriotism: if the generous Research Corporation were to withdraw, grasping Caltech would rush into the vacuum. "I know how repugnant it is for any right-thinking scientist to become embroiled in a discussion concerning priority of discovery. . . . However, California Technology is quite a 'powerful Katinka' and is out for both intellectual recognition and financial return whenever proper and possible. . . . Under these conditions might it not be possible to straighten up a little bit in your claims for patent priority?" Lawrence could scarcely deny this appeal from his benefactor for help against his rival. "It is entirely proper," he replied, "for us to look out for the commercial aspects of our work, if this can be done in a dignified and proper way." He had not counselled withdrawal from distaste for battling Caltech, but from conviction that the patented cyclotron was protection enough.[121]
Knight's strategy was to obtain an affidavit from Lawrence that he had had the idea of "irradiation" by deuterons before June 1933. That might allow patenting of (d,p); it might also require
[120] Knight to Poillon, 9 Jan 1933 (35/3); Lawrence to Knight, 28 Sep 1933 (35/2). The Corporation decided to file in Canada; Knight to Poillon, 19 Oct 1933 (35/2).
[121] Lawrence to Knight, 13 Sep, 9 Oct, and 23 Nov 1935; exchange with Poillon, 10 and 19 Oct 1935; all in 35/7.
Lawrence to claim discovery of artificial radioactivity. Knight's Washington correspondents arranged a meeting between Lawrence and the examiner, which they judged to be encouraging. Lawrence abandoned his application for radiosodium, substituted a claim for the (d,p) process, and swore that he had ordered the experiments of 1933.[122] The examiner rejected all claims based on the experiments and the affidavit and found a new objection: Cockcroft and Walton must have had some deuterons among their hydrogen ions; they therefore must have made something radioactive by (d,p); and consequently, by patent logic, they discovered without knowing it what Lawrence claimed as his own. The only way out seemed to be an affidavit from Lawrence's colleagues that he suggested the original bombardments with deuterons in order to search for radioactivity. This Lawrence was reluctant to seek—his colleagues might not like his running away with a patent on their joint work—but he would cooperate if necessary. He supplied a second affidavit, which the examiner again rejected as insufficient, since it did not declare (what would have been perjury) that Lawrence examined the product of the irradiation for radioactivity. Knight's correspondents judged that a sufficiently strong affidavit would win the day, but had the sense to doubt "whether upon the actual facts of the situation a fully satisfactory affidavit can be furnished."[123]
Lawrence had gone at least as far as he could. He wrote Knight: "The more I think about the matter the less enthusiasm I have for further endeavors to patent the process for producing the artificial radioactive substances." He retreated to his old position, much stronger in 1939 than it had been in 1935: "I feel that the cyclotron affords the only means of producing the radioactive materials in appreciable quantities; therefore with the cyclotron protected we have essential control of the matter." This time Poillon concurred, observing that the Research Corporation controlled
[122] Knight to Potter, Pierce, and Scheffler, 12 Nov 1935; to Lawrence, 23 and 30 Dec 1935; Potter et al. to Knight, 21 Apr 1936 and 23 Sep 1937; application no. 64,411, 17 Feb 1936, replacing no. 748,085 of 1934; all in 35/7.
[123] Knight to Lawrence, 27 May 1938; Lawrence to Knight, 7 Oct 1937 and 3 Sep 1938; Knight to Potter et al., 3 Oct 1938; examiner's statement, 22 Mar 1939; Potter et al. to Knight, 24 Mar 1939; all in 35/7.
not only the cyclotron, but essential features of the Van de Graaff generator too.[124]
Poillon had hoped to create and control a new industry of radio pharmaceuticals. Hence in the mid 1930s the Research Corporation invested redundantly in cyclotrons on the same principle that had built its cartel in the precipitation business: by expecting or requiring its grantees to assign improvements in the art to the Corporation. The theory is clear from the justification of a grant to Columbia to "enlarge the cyclotron . . . so that its field of application may be extended, and the equipment thus be made more effective for the preparation of artificial radioactive elements." In service of the same program, the Research Corporation supported work on the separation and application of biologically interesting stable isotopes, like C13 and O17 , at Columbia, and the ultracentrifuges of Jesse Beams at Virginia and of J.W. McBain at Stanford.[125] The purpose was highminded. As Poillon put it, "we do not in any way want to prevent scientists from having the free use of any discoveries that are made but if we can assess industry a reasonable sum, we will have just that much more to give to scientific research."[126] The methods, however, were those of the entrepreneur and the patent lawyer.
In 1940 it appeared likely that the Research Corporation would enjoy large royalties from its cyclotron patent. Several corporations, notably Westinghouse and American Cyanimid, deliberated building production cyclotrons for profit.[127] Radiophosphorus had shown promise in the treatment of certain sorts of leukemia. The annual cost of treating all Americans so afflicted was reckoned at between $200,000 and $500,000. Since the university cyclotrons in operation in 1940 could not supply the demand, let alone the requirements for other therapies and applications, commercial
[124] Lawrence to Knight, 26 Aug 1939 (35/7); Poillon to Lawrence, 5 May 1939 (15/18); Lawrence to Poillon, 27 Apr 1939, and exchange between Poillon and Knight, 5 and 10 Jul 1939 (RC).
[125] Research Corporation, "Minutes of meeting," 24 Apr 1940 (Columbia grant); cf. ibid., 25 Feb 1937 and 25 Mar 1938, and Lawrence to Poillon, 2 Jan 1938; all at RC. Infra, §8.3, for the competition between stable and radioactive isotopes.
[126] Poillon to Lawrence, 4 Nov 1935 (35/7).
[127] Cooksey to Aebersold, 11 Jul 1940 (1/9); W.B. Bell, American Cyanimid, to Lawrence, 27 Jul and 30 Aug 1940 (1/19); and, re Westinghouse, DuBridge to Lawrence, 28 Aug, and answer, 2 Sep 1940 (6/17).
production of radioisotopes under patents held by the Research Corporation seemed imminent and humanitarian. American Cyanimid hesitated only over the worry that universities might undersell commercial radioelements if they started to charge for the products of their cyclotrons. Both Poillon and Lawrence reassured Cyanimid that the demand would be so large, and the output of university cyclotrons available for therapy so small, that the commercial market would not be affected by the policies of university laboratories. In preparation for a windfall, the Research Corporation pushed Lawrence to patent certain cyclotron improvements.[128] The war ended these initiatives in two ways: by providing other lines of work for the interested parties and by creating, in the atomic pile, a much more efficient engine for the production of radioisotopes than the cyclotron. After the war, the Research Corporation wrote to all cyclotron laboratories to grant royalty-free licenses "for educational, scientific, experimental and research purposes." That amazed many. As the director of the Biochemical Research Foundation (Bartol) wrote in acknowledgement of this largesse: "I never knew there was such a patent."[129]
The Research Corporation had not cared to exercise its rights when the primary consumers of artificial radioelements were research teams in universities and hospitals. And, as Lewis had done with heavy water, Lawrence distributed the fruits of the cyclotron gratis throughout the world. To be sure, the product spread the fame of the machine that produced it; but the Laboratory made its gift in a true spirit of scientific cooperation. Lawrence had several reasons for not charging even the cost of production: he thereby retained the right to support only projects he thought worthwhile; he had to avoid giving the men in the Laboratory the impression that they were cogs in a business; and he wanted to repay in some measure the support he had received
[128] Letters to V. Bush from J.A. Fleming, 9 Sep 1940, and J.H. Lawrence, 10 Sep 1940 (15/18); Poillon to Lawrence, 20 Sep 1940, and response, 24 Sep 1940 (15/18).
[129] E.g., Joseph Barker to Sproul, 27 June 1947; E. MacDonald to Barker, 2 Jul 1947, quote (3/35); infra, §8.3, for leukemia therapy. Lawrence urged that even commerical firms be given royalty-free licenses; Lawrence to Barker, 30 Jan and 18 Feb 1946 (3/35).
from charitable foundations and public bodies.[130] The industry Poillon had envisaged did develop, after his patent expired. The first commercial cyclotrons for radioisotope production were made by the Collins Radio Company in the 1950s. In 1957 the former chief engineer at the Laboratory, William Brobeck, marketed cyclotrons for neutron therapy. By 1970 the annual sales of cyclotron-produced radioisotopes exceeded $3 million. It was but a small part of a big business—some $50 million a year—in radioisotopes for research, diagnosis, and therapy.[131] Poillon had the right idea but the wrong machine.
Other Players
Early in 1935 the chief Italian journal of physics, Nuovo Cimento , pointed out that Lawrence was behind Fermi's group in the discovery of Na24 . The Research Corporation likewise came late in the effort to patent it. On October 26, 1934, Fermi's group obtained an Italian patent covering activation by the absorption of fast or slowed neutrons and the products of the process, including radiosodium, as well. This violation of the physicist's ethos originated not with Fermi but with his patron O.M. Corbino, who had close ties to what high-tech industry then existed in Italy. "Age gave him wisdom," Mrs. Fermi writes, "[and] the boys were used to following his advice."[132]
But they, too, had been anticipated by "the inventor of all things."[133] In March 1934, a month or so after learning about artificial radioactivity and before Fermi's group had demonstrated the efficacy of neutrons, Szilard applied for a British patent on the "transmutation of chemical elements." His "invention," which he never reduced to art, had three parts: generation of neutrons to provoke reactions; separation of radioisotopes produced by the
[130] Lawrence to DuBridge, 14 June 1939 (15/26A). Probably no cyclotron laboratory charged for its products before the war; J.A. Fleming to V. Bush, 6 Sep 1940 (MAT, 25/"biophys.").
[131] Texas Ind. Comm., Texas giants , 6–7; Brobeck, interview by Seidel, June 1985 (TBL); IEEE, "1978 Conference," IEEE trans., NS, 26 (1979), 1703–32; Highfill and Wieland, ibid., 2220–3.
[132] A.P., Nuovo cimento, 12 (1935), 123–4; Segrè, Fermi , 83–5; L. Fermi, Atoms , 101, quote; Russo, HSPS, 16:2 (1986), 286.
[133] McMillan to Mann, 3 Jan 1952 (12/31).
(n,g ) process; and utilization of the heat liberated in the transmutation. Szilard's eccentric genius is displayed to full advantage in his method of obtaining neutrons. He planned to use deuterons accelerated by a high-tension device to create neutrons in collisions with light nuclei like beryllium or deuterium (he had made good use of the indications then accumulating of the d-d reaction that had misled Lawrence); he also proposed getting his neutrons from light, via (g ,n), and sketched an apparatus for making and absorbing photoneutrons.
The productivity of the transmutation evidently depends upon the number of neutrons at work. Szilard observed that if there exists a nucleus that when struck by a neutron liberates another without capturing the first, a very rapid buildup of a free neutronic population might occur. Mixing these hypothetical neutron multipliers with the material to be transmuted would increase the efficiency of transmutation; and a large enough sample of the material capable of sustaining the chain reaction (n,2n) would make a fine explosive. For the rest, Szilard proposed to separate isotopes made by (n,g ), which are chemically identical to their parents, by exploiting a process he did take the trouble to test.[134] His scheme as of June 1934, including provision for extracting power, appears in figure 4.2.[135]
Szilard considered assigning his first British patent on isotope and energy production to the Research Corporation in return for a grant for three years to continue research on the subject.[136] Instead, he licensed it to a relative of Brasch's, a Havana importer named Isbert Adam, in return for $15,000 in research support. (Szilard thereby made more money from radioactivity than the Research Corporation, even after subtracting the $7,000 he subsequently repaid Adam to reacquire the patents in 1943.)[137] In March 1936, when Szilard obtained a second patent on chain
[134] British patent application no. 7840, 12 Mar 1934, preliminary drafts, and supplements, 4 Jul and 20 Sep 1934, in Szilard, CW, 1 , 605–28, resulting in British patent no. 440,023, granted 12 Dec 1935.
[135] From supplementary application, 28 June 1934, resulting in patent no. 630,726, of 30 Mar 1936; Szilard, CW, 1 , 650.
[136] Szilard to Fermi, 13 Mar 1936 (Sz P, 17/197), and to Cockcroft, 27 May 1936, in Weart and Szilard, Szilard , 47–8.
[137] Szilard licensed patent no. 440,023 to Adam, Dec 1936 (Sz P, 29/311).
Fig. 4.2
Szilard's cornucopia of radioelements. Deuterons from the source 1 make
fast neutrons from the beryllium target 28, which spread through a sphere
3 composed of all elements that might multiply neutrons and that might
develop energy on absorbing neutrons. The tubes 107, 110, 111 contain a
coolant that delivers the heat of the reaction to an engine not shown.
Szilard, CW, 1 , 650.
reactions, he had reason to believe that multiplication of neutrons was possible. In his applications of 1934, he had imagined three sorts of interactions: a neutron multiplication (n,2n), a neutron conversion (n,2n ), and a neutron reduction (2n ,n), where 2n is a hypothetical heavy neutron with twice the mass of an ordinary one. In his definitive specification of June 1934, the basis of his patent of 1936, Szilard made the success of the chain reaction depend upon the existence of heavy neutrons, and offered indium, which in his experiments suffered an (n,4n ) reaction, as an element of the conversion type.[138]
He mobilized fellow Hungarian refugees Eugene Wigner and Michael Polanyi to procure the material needed to initiate a chain reaction. He acquired a cylinder of beryllium, and access to a big radium bomb in a London hospital, to make photoneutrons. He convinced himself that indium could give out at least one double neutron via (n,2n) or (n,2n ).[139] A trip to the United States provided leisure to weigh the whale he had hooked; and in March 1935 Szilard filed for an American patent on a large-scale transmutation process, similar to the earlier schemes, but with uranium and water (to slow the neutrons) as the neutron multiplier. All this was before the discovery of fission.[140] The responsibility for the chain reaction grew too heavy for Szilard to carry and in order to keep it secret he offered to assign the patent detailing it (his second British patent on transmutation) to the War Office. The official who examined the gift could see no value in it. Szilard had better luck with the Admiralty, which accepted his assignment in March 1936.[141]
As Szilard explained his actions to Fermi and to his British colleagues, he had never considered the patents to be his private property. He proposed to Fermi that they share responsibility for
[138] Szilard, CW, 1 , 643–6 (28 June 1934); memos of 13 and 28 Jul 1934 (Sz P, 29/309, 17/197); Weart and Szilard, Szilard , 39–40.
[139] Szilard to Wigner, 7 Aug 1934; to Lange, 6 Nov 1934; to Brasch and Lange, 12 Dec 1934; to Lindemann, 3 June 1935; all in Sz P, 17/197. The important part of the letter to Lindemann is also in Weart and Szilard, Szilard , 41–2.
[140] Szilard to Singer, 16 June 1935 (Sz P, 17/197); U.S. patent application no. 10,500, filed 11 Mar 1935, in Szilard, CW, 1 , 654–90.
[141] J. Combes to War Office, 8 Oct 1935, and director of navy contracts to Szilard, 20 Mar 1936 (Sz P, 17/197); Szilard to C.S. Wright, Admiralty, 26 Feb 1936 (Sz P, 44/476), in Szilard, CW, 1 , 733–4.
controlling a fund secured by the promise of their patents. "It must be awkward for any scientist to have a personal interest from such patents," Szilard wrote, "while other scientists, who also could have taken out such patents, refrain from doing so." As for the research the fund might support, Szilard did not see the wisdom of the course of the Research Corporation and its Berkeley client; "I personally do not think very much of producing radioactive elements for medical purposes and I should not like to be responsible for inducing manufacturers to embark upon such an enterprise at present."[142] (In that he was not entirely free from duplicity, since he wrote by the same mail to his patron Adam that the first priority was a systematic search for long-lived elements suitable for medical purposes). Nor did Szilard think much of the cyclotron. As he wrote to encourage Adam: "The artificial production of radioactive isotopes in California that you mention depends on a principle different [from mine], which I think is not susceptible of development and will have scarcely any commercial importance."[143]
For development of his more promising scheme, Szilard thought that he could do with perhaps 1,000 pounds sterling a year and, if Fermi came in, 5,000 pounds for three years, less than a sixth of Lawrence's rate of consumption.[144] Still the sum was not easy to raise. The agent of the Italian group, G.M. Giannini, agreed that the combined patents would make a nice set and professed an interest in cooperation; Segrè liked the idea of capitalizing the patents for a research fund, and for the researchers, "which would also indirectly advance science." But nothing came of it. Szilard continued to consider himself a disinterested broker.[145] The Italians preferred to make money and hoped for a
[142] M. Goldhaber to Szilard, 18 Mar 1936, in Weart and Szilard, Szilard , 44: "Of course, your intentions were misunderstood to be financial or otherwise unscientific." The idea of a patent pool for public purposes appears in Szilard's memo to himself of 13 Jul 1934 (Sz P, 29/309).
[143] Szilard to Fermi, 13 Mar 1936, in Szilard, CW, 1 , 729–30; to Segrè, 1 Apr 1936, ibid., 732; to Adam, same date (Sz P, 29/310), and 14 Oct 1935 (Sz P, 44/476).
[144] Szilard to Singer, 9 and 16 June 1935 (Sz P, 12/197); to Fermi, 13 Mar 1936, in Szilard, CW, 1 , 729–30; to Segrè, 1 Apr 1936, ibid., 731–2.
[145] Giannini to Szilard, 8 Mar and 20 Apr 1936; Segrè to Szilard, 21 Mar 1936; Szilard to Giannini, 8 Aug 1936, all in Sz P, 44/476.
time to set up an industrial concern; they turned down an option on the patents offered by Metropolitan-Vickers on Allibone's recommendation and ended by selling their European rights for $3,000 to Philips of Eindhoven, which had set up a nuclear section partly as a result of a visit from Segrè.[146] They failed to interest any American corporation in their radioactive technique. Still, they obtained more than they dreamt of in the 1930s when, after much haggling, the U.S. government, which had exploited their technique during the war, paid them $400,000 as "just compensation."[147] As for Szilard, he felt obliged to explain his altruistic policies to the major British physicists, and, when Fermi's group went its own way, to license Adam.[148]
There is another round to the story. In 1938 Szilard removed his headquarters from England to New York City, where he rightly expected to find greater scope for his schemes. He now concentrated on improving the neutron source. From data on neutron yields provided in papers from Berkeley and Rome, Szilard calculated that the energy that could be stored in transmuted radioactive atoms might be one hundred times the energy of the bombardment required to make the neutrons to make the transformations. Economics had given its blessing; only a little ingenuity was required to make a nuclear-powered airplane. "Perhaps we ought to think of new methods for producing really strong neutron beams." Szilard had proposed to Brasch to scale up a hightension machine to slam electrons into metal walls at 10 MeV. The resulting x rays would sire neutrons in profusion from a beryllium target.[149]
[146] Bakker to Segrè, 21 Jul 1935 (letter in Segrè's possession). Siemens of Berlin also began to take an interest in radioelements in 1935, in October, when it established a laboratory under Gustav Hertz to investigate production possibilities. Osietzki, Technikges., 55 (1988), 32–3.
[147] Allibone, PRS, A282 (1964), 451, and in Hendry, Cambridge physics , 171–2; Segrè, Fermi , 84–5. The American patent covered activation by slow neutrons; the Italian group tried also to patent all neutron activation, which Lawrence thought "ridiculous." Lawrence to Poillon, 24 Sep 1940 (15/18).
[148] Szilard to Cockcroft and to Rutherford, 27 May 1936, in Weart and Szilard, Szilard , 45–8; to Cockcroft, 21 May 1936 (Sz P, 17/197).
[149] Szilard to Brasch, 3 and 10 Jul 1937 (Sz P, 29/307); cf. Szilard to Adam, 2 Oct 1935 (Sz P, 44/476), on Szilard's earlier efforts to mobilize Brasch.
For money Szilard appealed to Lewis Strauss, a Wall Street financier with an interest in radiation therapy for cancer, who thus entered on his controversial career in atomic energy. As a light inducement to cooperation, Szilard offered to give to any taxexempt nonprofit corporation Strauss might wish to set up for producing radioelements a nonexclusive license to exploit whatever of his rights he had not sold to Adam. Strauss tried to enlist Westinghouse, General Motors, and General Electric; but all that came of it was an introduction to another supplicant, "whose friendship for the following twenty years was one of the finest experiences of my [Strauss's] life." That was Lawrence, who this time got nothing from Strauss. The future friend had decided to support Brasch and Szilard, if only a suitable place for Brasch's experiments could be found. One was. Toward the end of 1938 the ever-acquisitive Millikan offered space at Caltech, on the understanding that it would cost him nothing.[150]
Brasch intended to reach for 15 MV, which Millikan thought "exceedingly interesting and thrilling," and also expensive, over $100,000. Strauss doled out his money in droplets; Brasch complained that he could not exist on "homeopathic doses" of dollars, and raised the estimate to $200,000; by 1940 the adventure had come to an end nowhere near its goal.[151] Meanwhile fission had been discovered, and the royal road to atomic energy. Szilard kept Strauss apprised of progress by telegram, although they were almost neighbors, and asked his benefactor to find him another. Szilard had in mind Alfred Loomis, a retired investment banker and a first-rate amateur physicist, who soon became one of Lawrence's main advisors and supporters.[152] Szilard wished Loomis to help underwrite the cost of experiments he planned to try at Columbia University, where he had a guest appointment, to determine how fission might be exploited for atomic energy. To complete the circle, the new émigré professor of physics at Columbia, Enrico Fermi, was then engaged in the same line of work. We shall return to their unequal competition.
[150] Szilard to Strauss, draft, n.d., and to Adam, 16 Nov 1938 (Sz P, 29/306); Strauss, Men and decisions , 163–5.
[151] Brasch to Szilard, 27 Jan 1939, and n.d. (Sz P, 29/306); Millikan, quoted by Strauss, Men and decisions , 168.
[152] Szilard to Strauss, 28 Feb 1939, and telegrams, Feb–Apr 1939 (Sz P, 17/198).