Pest and Beneficial Insects Associated with Agriculture and Riparian Systems[1]
Vernon M. Stern[2]
Abstract.—Vast changes have occurred in the California landscape over the past 100 years. In agriculture, many of these changes favor the buildup of pest populations over biological control. The manipulation of pest populations is a complex study in applied ecology.
Introduction
The species of insects, spider mites, and their close relatives far outnumber all other species of animals in the world. Insects are a complex group, with complicated life histories and interrelationships with other animals and plants. There are more than one million insect species, but only a relatively small number are pests. In the United States there are several thousand such species. These range from those that appear sporadically to major "key" pests that appear annually (Knipling 1979). These pests cause billions of dollars in losses each year in the United States (US Department of Agriculture 1965).
There are also thousands of insect parasites and predators that are natural enemies of pest species. Many crops, such as alfalfa, have a rather large complex of these biological control agents that feed on actual pest species, as well as feeding on an even larger number of potential pests. Often these potential pests can be very important in providing alternative hosts for parasites or food sources for predators. In this way potential pests help to stabilize and maintain populations of natural enemies when the pest population is at a low level, absent, or otherwise unavailable.
There are, of course, many "indifferent" species present in natural and agroecosystems. Their population numbers are not necessarily related to the numbers of pests or natural enemies. These include the scavengers, decomposers, pollinators, and other forms that may provide some useful function in agroecosystems, as well as in riparian and other natural systems. An example of these is the insects that provide food for birds and fish. However, the precise function of the vast majority of "indifferent" species is generally unknown.
In California, the replacement of natural communities with monocultures of agriculture has caused general faunal impoverishment, while certain species of phytophagous arthropods have become extremely abundant (Smith and Allen 1954). Many of these pest species have developed a high degree of mobility (Southwood 1966). Being good flyers, they often colonize the disrupted agroecosystem ahead of their natural enemies (van den Bosch and Telford 1964).
Burnett (1960), Odum (1971) and many others comment that as biotic complexity increases, particularly with reference to the number and kinds of trophic or feeding interactions, the stability of an agroecosystem will increase. An opposing, although not widely accepted, viewpoint of this ecological "dogma" is taken by van Emden and Williams (1973). They argue that species diversity does not necessarily cause greater stability. However, there are many examples of crop environment diversification which do make it more favorable to natural enemies and less favorable to a pest (Stern etal . 1976).
In general, rich, diverse, biotic complexes should be fostered and maintained. However, the function of the various elements must be understood before they can be intelligently employed or manipulated. The fact that most crop monocultures are threatened annually by pests and the diverse climax vegetation of many natural environments is little harmed has led to a general assumption that maximum diversity is desirable in all agricultural areas. It is thought that this will preserve the continuity, stability, and diversity inherent in the natural environment. There is an element of truth here, but it can be overstressed. In some cases, the "semi-natural" vegetation that adjoins crops can provide overwintering sites, sheltering places, and food for certain crop pests. Such vegetation may also benefit natural enemies if it supports their
[1] Paper presented at the California Riparian Systems Conference. [University of California, Davis, September 17–19, 1981].
[2] Vernon M. Stern is Professor of Entomology, University of California, Riverside.
alternative hosts, but this will not automatically provide a marked increase in biological control. Much depends on whether these benefits to the natural enemies counteract the benefits to the pest population. Once the delicate stability of a climax vegetation has been disturbed by man, even if only slightly, the vegetation, although still complex, may have been sufficiently altered to provide greater benefit to a pest in relation to the natural enemies that previously regulated it (Billiotti etal . 1968). Obviously, the manipulation of insect pest populations is a complex study in applied ecology.
Pests of California Agriculture
The hundreds of insect and spider mite pests attacking California agriculture have come from two sources. These are native species and exotic or introduced species.
Introduced Pests
Introduced pests comprise about 66% of our arthropod pests. Two problems of immediate concern are the Mediterranean fruit fly (Ceratitiscapitata ) and the gypsy moth (Lymantriadispar ). The Mediterranean fruit fly was apparently transported to California in 1979. In some way adults or maggots inside infested fruit escaped detection at quarantine inspection stations. This devastating fly is one of the most serious entomological problems ever encountered by California agriculture. It attacks many crops and will cost many millions of dollars for various types of chemical control and in food loss each year if not eradicated.
On the other hand, the gypsy moth was intentionally brought to the United States by a Professor L. Trouvelot from Harvard. In 1869 he collected gypsy moth egg masses in France and brought them to the United States. His intention was to breed the gypsy moth with the silkworm to overcome a wilt disease commonly plaguing silkworm cultures in France and Italy. For whatever reason, he laid the egg masses on a window ledge, and evidently the wind blew them away.
Professor Trouvelot then published a notice, warning other citizens to be alert for the insect. The incident was forgotten until numerous shade and forest trees in the area were completely defoliated by the voracious caterpillars. Harassed by his irate neighbors, the professor left Harvard and headed west into entomological history.
By 1891 about 518 sq. km. (200 sq. mi.) north and west of Boston were infested. Even though the female moths cannot fly, the insect has steadily spread and now infests nearly all of the New England states and has extended into the Ohio River drainage system.
Through 1972 the gypsy moth had defoliated more than 810,000 ha. (2 million ac.) of forestland and killed over 5 million trees. The leaves of over 500 species of trees and other plants are eaten by these caterpillars. Important hosts that can be completely defoliated along riparian zones, in and around parks, homes and streets, and in forestland areas include alder, all species of oak, gray birch, basswood, willow, river birch, all species of poplar, box elder, hawthorne and apple. Less defoliation will occur on all species of maple, yellow birch, and elm. The larger caterpillars will also attack all species of pine, hemlock and spruce (Brown 1978).
The first gypsy moth infestation was detected in California in 1976 (Eichlin 1981). Over 400 egg masses were found in Santa Clara County. The California Department of Food and Agriculture (DFA) conducted an apparently successful eradication program against the infestation using two aerial applications of the insect growth regulator diflubenzuron (Hoy 1982).
During 1981, 41 male gypsy moths were trapped in Santa Barbara County. Lesser numbers were trapped in other southern and northern California counties—Los Angeles (3 moths), Marin (7), San Diego (3), Santa Cruz (2), and Ventura (2). Capture of male moths in sex-lure pheromone traps does not prove that the gypsy moth has become established in an area, because they may have developed from eggs or pupae brought into the state from infected areas in the eastern United States. However, intensive surveys in Santa Barbara during the fall and winter (1981–82) revealed four egg masses, indicating that a breeding population of the gypsy moth exists there (ibid .).
Professor Trouvelot's seemingly harmless 1869 venture occurred before the Plant Quarantine Act was passed in 1912. This federal legislative act established regulatory measures dealing with insects and plant diseases. All the states now have regulatory measures in force forbidding the movement of certain plants into or within the state, at least until such plants have been inspected or fumigated, or both. In many cases reinspection is required at the point of destination. Further, it is illegal to ship nursery stock anywhere in the United States unless it is accompanied by a certificate of inspection, stating that it has been found apparently free from certain seriously destructive insect pests and plant diseases.
However, with national and international passenger and cargo aircraft (including military aircraft) constantly entering California, as well as a domestic population coming into California as tourists or to live, it is a monumental task to keep out pests of agriculture and natural ecosystems.
Today, California has hundreds of examples of established exotic insect pests. A few of them are: Egyptian alfalfa weevil, pink bollworm, green peach aphid, codling moth, peach twig borer, Oriental fruit moth, citrus red scale, and
olive scale. In addition, there is a wide array of plant pathogens, weeds, and nematode pests that have been inadvertently introduced. The best estimates from some of the author's colleagues are that the vast majority of plant pathogens, nearly all of our weeds, and most of the nematode pests were brought into California from other areas.
The successful establishment of hundreds of exotic pests ties in perfectly with California's wild mosaic of climatic conditions. These range from the hot Imperial Valley, where cotton growers fight the pink bollworm (introduced in 1965) with 10 to 12 spray applications each season, to the cold winters in Modoc County, where cattlemen spray their alfalfa once or twice each spring to control the alfalfa weevil (introduced in the 1930s). The wide array of climates also gives rise to more than 230 commercial crops grown in the state, providing an ample variety of food for pest insects.
Native Pests
When the first Spanish explorers entered the San Joaquin Valley in the late 1700s, they found a large population of Yokut Indians existing on an abundance of elk, antelope, fish, tule roots, acorns, pine nuts, and other seeds. Two huge lakes, Buena Vista (formed by the Kern River) and Tulare (formed by the Tule and Kaweah rivers), and their surrounding tule marshes covered most of the lowlands, at least in the spring months (Kahrl 1979). Large areas of grassland and oak savannah, together with smaller amounts of saltbush desert, chaparral, and riparian communities, were essentially undisturbed by man. A number of insects we know today as crop pests—the western yellow-striped armyworm, alfalfa caterpillar, lygus bug, western spotted cucumber beetle, grape leafhopper, corn earworm, salt marsh caterpillar, several grasshoppers, and a number of other species occurred there, but they could not be considered pests because no agricultural crops existed in the valley.
These insects were greatly influenced by the seasonal occurrence of rain and the limited distribution of native annual vegetation. None of the Mediterranean winter annual herbs and grasses, such as bur clover, filarees, wild oat, and foxtail, were present.
In 1836 the first cattle ranch was established by the Spanish in the northwest fringes of the San Joaquin Valley; animals were produced largely for their hides. The discovery of gold and the rapid influx of settlers from the eastern United states created a demand for beef and other food. The period of 1850–70 was one of huge cattle holdings. Overgrazing began to take its toll, especially in drought years, and the introduction of Mediterranean grasses and forbs changed the composition of the range. The white man had now developed huge pastoral agroecosystems and the indigenous Indian had virtually disappeared.
The discovery in the 1850s that the winter and spring rains were sufficient to produce tremendous crops of wheat brought an era which lasted until about 1890. Huge grainfields displaced extensive areas of native grasslands.
The establishment of railroads also permitted the development of general agriculture along the rivers where water was available. Irrigation systems had small beginnings and were continually threatened by problems involving riparian rights to water, financing, land frauds, large corporate landholdings, and state laws concerning water rights and irrigation districts (ibid ).
With the introduction of extensive irrigation systems, using water from snowpack runoff and deep wells, the grasslands and alkali deserts of the San Joaquin Valley were transformed into an intensive irrigated agriculture. Along with grains and alfalfa came tree fruits (deciduous and citrus), grapes, cotton, melons, sugar beets, rice, and vegetables. A variety of native insects found the lush irrigated fields or orchards an ideal haven. An abundant food supply was now available year-round, and their period of increase was no longer confined to the spring.
Indeed, the corn earworm (Heliothiszea ) (fig. 1) was just another night-flying moth in the ancient pristine habitat. It undoubtedly dispersed to lay eggs on various annuals germinating from the winter and spring rains. When the rains ceased and the annuals set seed and dried up, there was probably high mortality of the younger larval stages. The moth population emerging from cocoons retracted to riverine and marsh areas to complete two or three summer generations on host plants near water. Undisturbed by today's chemicals, parasites and predators undoubtedly took a heavy toll of the summer generation larvae.
Today, with over 3.6 million hectares (9 million acres) of lush, irrigated farm land and a wide variety of commercial host crops, the native corn earworm is now "King of the Hill;" the scourge of vegetable growers; "number one" agricultural pest in California. It costs growers $80–100 million in chemical control and crop loss annually (California Department of Food and Agriculture 1977).
All of these changes have had an impact on the buildup of insect pest populations in areas of intensified agriculture. There are, of course, many ecological reasons why pest problems are more severe in intensified agriculture than in natural communities (table 1). Indeed, with our intensified production of food, fiber, and forage, we have created our own arthropod competitors.
Man in the Environment
We must recognize that all organisms are subjected to the physical and biotic pressures of
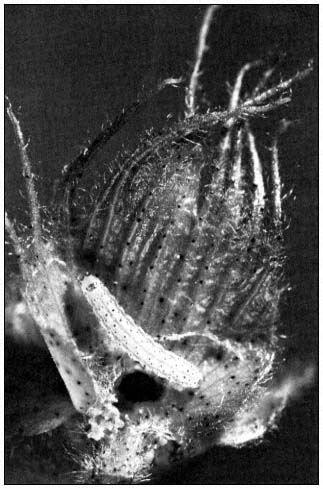
Figure 1.
Corn earworm (Heliothis zea ) larva feeding
on an immature cotton floral bud.
the environments in which they live, and these factors, together with the genetic makeup of the species, determine their abundance and existence in any given area. Without natural control, a species which reproduces more than the parent stock could increase to infinite numbers. Man is subjected to environmental pressures just as other forms of life are, and he competes with other organisms for food and space.
Utilizing the traits that sharply differentiate him from other species, man has developed a technology permitting him to modify environments to meet his needs. Over the past several centuries, the competition for food and space has been almost completely in favor of man, as is attested by the decimation of vast vertebrate populations, as well as populations of other forms of life. But, while eliminating many species as he changed the environment of various regions to fit his needs for food and space, a number of species became his direct competitors. Today, as the human population continues to increase and civilization to advance, man numbers his arthropod enemies in the thousands of species (Knipling 1979).
Arthropod pests of agriculture are those species present in a crop in sufficient abundance to reduce the quality and/or quantity of food, forage, or fiber. The numbers of a pest may be in balance with their arthropod predators and parasites. However, at various times the pest population level may be sufficiently high to cause crop loss. Some control measure is then required to decrease its numbers. During the period of crop growth, pesticides are often the only way to prevent crop loss or decreased marketability of the product. Fruits and vegetables present the most serious marketability problems because of state and certain federal regulations relating to
| ||||||||||||||
| ||||||||||||||||||||||||||||||||
product quality. Consumers are also involved, because they usually avoid buying food that shows signs of any type of insect damage which spoils the appearance of the product, even though there may be no nutritional loss to the product.
The increase to pest status of a particular species may be the result of a single factor or a combination of factors. The most significant factors of the last century are discussed below (Stern etal . 1959).
First, by changing or manipulating the environment, man has created conditions that permit certain species to increase their population densities. For example, when alfalfa was introduced into California in the 1850s to feed horses, the alfalfa butterfly (Coliaseurytheme ) (fig. 2), which had previously occurred in low numbers in native legumes, found a widespread and favorable new host plant in its environment. It soon became an economic pest.
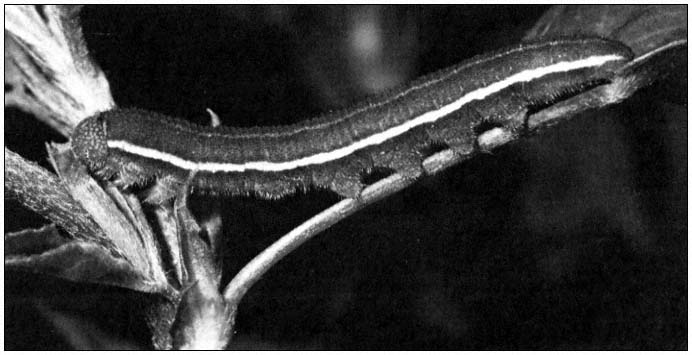
Figure 2.
Alfalfa caterpillar (Colias eurytheme ) larva consuming alfalfa. This $800 million forage
crop is the main food for California's multi-billion dollar dairy and beef cattle industry.
A second way in which arthropods have risen to pest status has occurred by their being transported across geographical barriers while leaving their specific predators, parasites, and diseases behind. The increase in importance through such transportation is illustrated by the cottonycushion scale (Icerya purchasi ) (fig. 3). This scale insect was introduced from Australia on acacia in 1868. Within the following two decades, it increased in abundance to the point that it threatened economic disaster for the entire citrus industry. Fortunately, the timely importation and establishment of two of its natural enemies, a lady beetle (Rodolia cardinalis ) and a parasitic fly (Cryptochaetumiceryae ), resulted in the complete suppression of I . purchasi as a citrus pest.
A third cause for the increasing number of pest arthropods has been the establishment of progressively lower economic thresholds. For example, prior to the discovery of the insecticidal properties of DDT and development of other organic pesticides, the blotches caused by lygus bugs feeding on an occasional lima bean were of little concern. However, with a cheap and readily available method of chemical control of lygus bugs for the first time ever, and with the emphasis on product appearance in the frozen food industry, a demand was created for a near-perfect bean. For this reason, lower economic thresholds were established and lygus bugs came to be considered a serious pest of lima beans.
A fourth cause has been the indiscriminate use of pesticides. As mentioned, the corn earworm (Heliothiszea ) is a native insect species and the single most damaging pest in the state. In the late 1950s through the 1960s, cotton was treated widely in the San Joaquin Valley for lygus bug control. Chemical treatments were often started in early June and continued through August. The corn earworm frequently increased to devastating numbers in August and September. From 1967 through 1972, University of California cotton research entomologists demonstrated that lygus bugs were not a pest of cotton after late
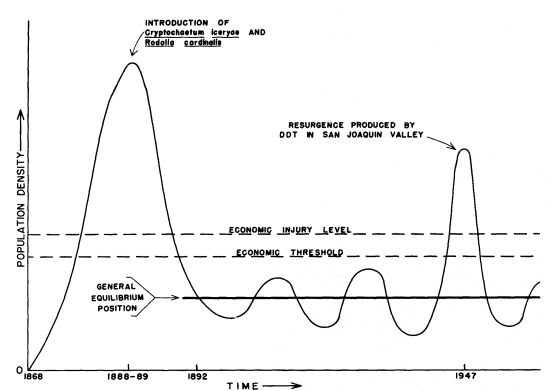
Figure 3.
Schematic graph of fluctuations in population density
of the cottony-cushion scale (Icerya purchasi ) on citrus
from its introduction into California in 1868. Following
the successful introduction of two of its natural enemies
in 1888, it was reduced to noneconomic status except
for a local resurgence produced by DDT treatments.
July. This and other knowledge reduced lygus bug treatments by nearly 50%, and as a consequence, the corn earworm has been reduced to minor pest status on cotton in the San Joaquin Valley.
Pest Interactions between Riparian Systems and Intensified Agriculture
Pierce's Disease
Pierce's disease (PD), which kills grapevines, is caused by a bacterium. This pathogen is spread by a family of leafhoppers known as sharpshooters. The disease had been present in California since the 1880s (Winkler etal . 1949). It initially broke out in southern California, destroying 14,000 ha. (35,000 ac.) of grapes. In the Central Valley the disease was first noted about 1917; from 1933 to 1940 a major outbreak devastated many Central Valley districts. Today the most dramatic vineyard losses to PD occur in the Napa Valley and parts of the San Joaquin Valley. During severe epidemics, losses to PD may require major replanting.
For many years PD was thought to be caused by a virus (ibid .). However, in 1973, University of California researchers, using the electron microscope, discovered the bacterium in infested grapes. This bacterium infects many different plant species in addition to grapevines. The host plants serve as reservoirs from which the sharpshooter vectors pick up PD bacterium for transmission to grapes. Many host plants of the bacterium also are important food or egg-laying places for the sharpshooter vectors. Some of these include Bermuda grass (Cynodon dactylon ), watergrass (Echinochloacrusgalli ), blackberry (Rubusvitifolius ), willow (Salix spp.), and stinging nettle (Urticaholosericea ).
Different grape varieties show different sensitivity to PD. Some of the more sensitive varieties include Mission, Pinot Noir, Pinot Chardonnay, and Barbera. Moderately resistant are Gray Riesling, Petit Sirah, Sauvignon Blanc, Cabernet Sauvignon, and Napa Gamay. Relatively resistant varieties are Thompson Seedless, White Riesling, Chenin Blanc, and Ruby Cabernet. Unfortunately, a number of California's varietal wine grapes are moderately to very sensitive to the disease (Purcell 1981).
Thus, the answer to the PD problem is not quite so simple as ripping out the more sensitive varieties and replanting with resistant varieties. This method of control is often used with field and vegetable crops which are planted for a single season. However, these latter crops are not required by law to carry a varietal label as are varietal wines.
Management of the Disease
Most dispersal of PD seems to develop directly from outside source areas and is then vectored to individual grape vines within vineyards. Studies in both coastal and interior areas have shown that rigorous removal of infected vines is not beneficial in reducing the spread of PD within a vineyard, although removal and replanting may be necessary to keep up vineyard productivity. Therefore, the only way to reduce or prevent PD spread through vector control is to prevent sharpshooters from entering vineyards.
Insect Vectors of Pierce's Disease
The green-headed sharpshooter (Draeculacephala minerva ) and red-headed sharpshooter (Carneocephala fulgida ) are the primary vectors of PD in the Central Valley. The two sharpshooters feed mainly on grasses, where they pick up the bacterium. These grasses occur commonly along irrigation ditch banks, roadways, pastures, and irrigation drainage sumps. In alfalfa fields, orchards or field-crop areas, it is the grass weeds growing in or at the margins of the crops that result in increased sharpshooter populations. If the PD bacterium is present, vineyards adjacent to such locations may suffer a high incidence of PD (ibid .). Unfortunately, control of weed areas or weedy alfalfa plantings may not be the prerogative of the vineyard owner. However, the owner can eliminate weed grasses on his or her own property to help reduce PD.
Insecticidal treatment is only temporarily effective against adult sharpshooters and of little value overall in the Central Valley. There is a considerable overlap of generations; eggs are present inside protective leaf tissues of host plants from January through the fall.
Coastal Areas—Napa Valley
The blue-green sharpshooter (Graphocephala atropunctata ) (fig. 4), unlike the grass-feeding sharpshooters of the Central Valley, will feed and reproduce on many plants. However, it prefers woody or perennial plants such as wild grape, blackberry, elderberry, and stinging nettle. The blue-green sharpshooter is most common along streambanks or in ravines or canyons that have dense growth of trees, vines, and shrubs, where it feeds on succulent new growth in areas of abundant soil moisture and shade. It is seldom found in unshaded dry locations.

Figure 4.
Blue-green sharpshooter (Graphocephala atropunctata ),
an important vector of Pierce's disease (PD) of grapes
in coastal areas of California.
Vineyards in the immediate vicinity of environments favorable to this sharpshooter are frequently PD "hot spots" with a very high incidence of the disease. Unfortunately, removal of the host plants carrying the bacterium is usually impractical because often they provide refuges for wildlife, protect against erosion and flood damage, and contribute substantially to the beauty of the countryside.
To combat the blue-green sharpshooter, which has only one generation per year, insecticide applications of dimethoate have been used in early spring, before the leafhoppers move into the vineyard. At that time, feeding and most flight activity near vineyards is concentrated in relatively small and well-defined locations. Treatment consists of applying the pesticide to a band of native vegetation about 15–30 m. (50–100 ft.) wide along the vineyard edge. At the same time that the natural vegetation is treated, adjacent portions of the vineyard should be treated if new shoot growth is longer than a few centimeters (ibid .).
While blackberry acts as a host to the PD bacterium in the Napa Valley, this riparian plant species can be beneficial in other ways, including its influence on the grape leafhopper.
Grape Leafhopper
The grape leafhopper (Erythroneuraelegantuala ) (fig. 5) is the most common insect pest of grapes in the San Joaquin, Sacramento, and Napa valleys. Before the development of organic insecticides in the 1940s, severe damage occurred in some years, followed by periods of low populations. Now, with more effective pesticides, losses are primarily due to the cost of treatment plus the side effect of secondary outbreaks of other insect and spider mite pests resulting from the chemical treatment. A good example occurred in the 1960s when the leafhopper developed resistance to DDT and other chemicals. Growers began using the pesticide Sevin for leafhopper control. This material often caused severe outbreaks of spider mites, and in many cases the mites were more damaging to the grapes than the original leafhoppers.

Figure 5.
Grape leafhopper (Erythroneura elegantuala ) is the most common and
most frequently treated grape pest in central and northern California.
Injury
Both adults and nymphs of the grape leafhopper have piercing mouth parts with which they puncture leaves and suck out the contents. Heavily damaged leaves lose their green color, dry up, and fall off the vine. In addition, in table grapes excessive fruit spotting from the leafhopper excrement can lead to unmarketable fruit before any effect on yield is noted. The crop is then sold for crushing, which is far less profitable than table grapes.
Natural Control
The important natural enemy of the grape leafhopper is a tiny, almost microscopic wasp Anagrusepos (fig. 6). This tiny wasp lays its eggs in the eggs of the grape leafhopper, resulting in the death of the leafhopper egg. These parasitic wasps are particularly valuable because of their amazing ability to locate and attack grape leafhopper eggs. Also, their short life cycle permits them to increase far more rapidly than can leafhopper populations. Their nine to 10 generations during the grape season make them capable of parasitizing 90–95% of all leafhopper eggs that are laid after July (Jensen and Flaherty 1981).
Anagrusepos overwinters on wild blackberries (Rubus spp.) on which it parasitizes the eggs of noneconomic, harmless leafhoppers (Dikrella spp.). These overwintering wasp populations tend to be along rivers that have an overstory of trees sheltering both wild grapes and wild blackberries. When the blackberries leaf out in February, the lush, new foliage apparently stimulates heavy oviposition by the Dikrella leafhoppers. The Anagrus populations increase enormously in these eggs, so that by late March and early April there is widespread dispersal of the newly produced Anagrus adult females. Fortunately, their dispersal occurs at the same time that grape leafhopper females begin to lay eggs. Vineyards located within an 8- to 16-km.

Figure 6.
Adult parasite (Anagrus epos ) of grape leafhopper eggs.
The male, shown above, is blackish-brown, while the
female is straw colored. This microscopic wasp often
reduces the grape leafhopper to nonpest status in
vineyards adjacent to riparian systems.
(5- to 10-mi.) range of an overwintering population will usually benefit immediately from the immigrant parasites. Vineyards distant from actual blackberry refuges may not show Anagrus activity until midsummer or later.
Efforts to establish blackberry refuges near vineyards in Tulare County have been only partially successful. This is attributed to the lack of a sheltering overstory of trees as would normally occur in riparian zones. In full sun, blackberry plantings gradually become dry thickets, with only surface foliage that becomes tough and leathery. Dikrella , wintertime host of Anagrusepos , is a shade- and moisture-loving insect that breeds on leaves inside blackberry vines were it is generally cooler and more humid. Old mature thickets, therefore, become less desirable Dikrella habitats and are, consequently, poor producers of Anagrus . A vineyard located within a natural dispersal area will, therefore, receive early important activity from the Anagrus parasites.
Where blackberries occur naturally, the commercial vineyards in the vicinity are not seriously troubled by grape leafhopper populations, and parasitism by Anagrus begins early in the season. This situation was first evident from studies in the Napa Valley, where blackberries are common, as well as in vineyards near the Stanislaus River, in the northern San Joaquin Valley. Certain locations along the Kings, Kaweah, and St. Johns rivers are also excellent sites for Anagrus where blackberries are common.
By contrast, the vineyards in the southern San Joaquin Valley are established in locations that are virtually reclaimed desert. They are miles from naturally occurring blackberries and chronically suffer from excessively high leafhopper populations. Parasitism by Anagrus does not occur until late in the summer, if at all.
Doutt and Nakata (1973) concluded that a vineyard situated within the predictable early dispersion range of Anagrus from overwintering sites can reasonably expect the parasite activity to be a substantial mortality factor on the first two broods of leafhoppers. This element is important in pest management programs of vineyards in the area, and any practice disruptive to this particular host/parasite system should be avoided.
Grapeleaf Skeletonizer
The western grapeleaf skeletonizer (Harrisina brillians ) (GLS) is an introduced pest (fig. 7). It was first found in California in 1941, near San Diego. In canyon areas, wild grapes (Vitisgirdiana ) were severely defoliated; in a short time GLS became a serious pest in commercial vineyards. By 1943, crop loss in some vineyards reached 90%, but the average was a loss of about 50%.
A state-imposed eradication program, using cryolite dust, was tried, but it was not successful. Meanwhile, emphasis was placed on biological control. University of California entomologists at Riverside imported parasites from Mexico and Arizona. A parasitic wasp, Apantelesharrisinae , and a parasitic fly, Ametadoria (= = Sturmia ) harrisinae , were soon established. A granulosis virus that attacks GLS larvae was soon found in GLS-rearing laboratories in Arizona and in San Diego County.
By 1961, GLS was found on backyard grapes at Kerman (Fresno County). In spite of another try at eradication, by 1975 devastating infestations could be found in central and northern California on wild grapes in riparian systems, on backyard grapes, and in commercial vineyards. It has spread slowly in the Central Valley because GLS adults tend to remain in the area of their larval development (Stern etal . 1981).

Figure 7.
Larvae of the western grapeleaf skeletonizer
(Harrisina brillians ) feeding on a grape leaf.
To deal with these new infestations, Apanteles and Ametodoria parasites from San Diego County were introduced, and explorations were made for new parasites from outside of California. So far only the parasitic fly appears to have become established, and this only in Siskiyou and Shasta counties where grapes are not commercially important.
Injury
Leaf damage continues to increase through the season in untreated GLS populations. Second and third generations (there are three per year) have defoliated entire vineyards in the central San Joaquin Valley. If vines are heavily or completely defoliated before harvest, fruit maturity can be adversely affected. Heavy defoliation in mid-summer usually destroys the entire crop. Defoliation after harvest is less damaging, but it can affect the food reserves of the vine and weaken it for the following year. At present, biological control is inadeqate and growers have no recourse other than to treat GLS infestations.
Riparian Systems as Biological Controls
When wild grape foliage is abundant, adult GLS moths tend to remain in the area and flitter about in the process of mating and laying eggs. The conspicuous bluish-black moths are caught easily by hand. However, when larvae have defoliated the wild grapes in a spot, adults emerging from the cocoons of these larvae change their flight habits. In seeking out new egg-laying sites, they become very swift fliers. With this unusual flight habit on the part of the moth, wild grapes along riparian zones are a constant source of infestation for commercial vineyards in the area.
On the other hand, the wild grapes along the rivers, streams, and creeks east of Visalia have been extremely beneficial in the attempt to establish biological control agents of GLS. These areas, some in permanent pasture, are never treated with insecticides which would possibly wipe out the released GLS parasites. This author has taken advantage of these riparian systems in cooperation with the private landowners for research projects. None of them grow grapes. One cooperator has walnut groves, another is a dairyman, and still another raises beef cattle and is a descendent of a family that homesteaded and bought property along the Kaweah River in the 1880s.
When the GLS parasites become firmly established in riparian vegetation, they can then spread by themselves into commercial vineyards. It might be noted that about 20% of the vineyards in the Central Valley are never treated with insecticides.
Even more promising, in terms of biological control of GLS, has been the work with the granulosis virus of GLS. During the mid-1950s, Edward Steinhaus, then Professor of Insect Pathology, University of California, Berkeley, purified about one-fourth teaspoon of the GLS virus and placed it in a refrigerator. In 1979 this small bit of virus was sent to the author at Riverside. The virus was tested in Tulare County on single grape leaves containing early larval stages of GLS. The virus proved to be just as potent as when Professor Steinhaus put it in the refrigerator 25 years earlier. Following this, the virus was propagated, and field tests were conducted in 1980. The virus was just as effective on GLS as commercial pesticides. Equally important for these biological control efforts was the effect of the virus at low dosages. These data suggested that the virus could be incorporated as a biological control and could contaminate the GLS population in the San Joaquin Valley. In this study the desired effect is not to kill the GLS larvae outright. Instead, the aim is to apply a sufficiently low virus dosage as to permit the infected larvae to survive so the adults will become carriers of the virus and spread it through the valley.
Adults arising from such larvae are one-half normal size. In 1981, it was found that some of these moths do not lay eggs. About 75% of the eggs that are laid do not hatch. Larvae that do hatch have abnormal feeding habits and eventually all die in the third larval stage. This gives 100% control from exposure to the virus in the previous generation.
During the summer, riparian areas were sprayed with low dosages of the virus. A unique and key feature of the GLS granulosis virus is
that it attacks the gut of the larvae. This gives rise to diarrhea which contains millions of virion particles. Other insects crawling over this material will pick it up on their legs and help spread it through the system.
Conclusion
In little more than a century, the land- and waterscape of California has been remade through construction of a great network of artificial lakes and rivers. However, the present water system and its domestic, agricultural, and industrial use is far more than these physical elements. It is also constructed of the legal and institutional structures that Californians have erected to govern water use and the social and economic development it has helped to foster. The money invested has led to the transformation of grassland prairie, as well as arid and semiarid land, into cities, towns, and irrigated cropland and has made California the most populous and agriculturally productive state in the nation.
The past changes as well as current and proposed changes in land use and waterscape are disturbing to a good number of Californians, and rightly so. The original grassland prairie, marshlands, and forests of willow, oak, cottonwood, and sycamore along rivers and streams, and their accompanying flora and fauna have largely disappeared. To resurrect a portion of the original habitat present when the Spaniards and settlers from the eastern United States arrived would require huge expenditures of public funds. To complicate matters even more is the vast array of public and private agencies (about 3,700) with administrative authority to respond to the political, social, and economic rights of various citizens with respect to water supply, delivery, use, and treatment (Kahrl 1979).
Strict conservation and maintenance of existing riparian areas is certainly needed. Limited restoration of riparian systems is also possible and can be accomplished through the legal process. This mainly concerns private donations in perpetuity of deeded land to an institution, such as the University of California, to remain as or to return to natural riparian habitats. Good examples occur in southern California through the efforts of Dr. William W. Mayhew, Professor of Zoology, University of California, Riverside. At present, donations of deeded land to the University far exceed those that the state legislature and the general public would be willing to purchase outright.
Aesthetic values aside, much remains to be learned of the beneficial as well as adverse effects of crop plant diseases, pest and beneficial arthropods, birds and other flora and fauna associated with riparian and agricultural systems. Riparian areas which harbor specific agents detrimental to adjacent agricultural crops will often have another array of beneficial agents affecting the same or another crop. Long-term ecological studies are needed toelucidate these relationships, and special funding to the University of California from the state legislature for this specific purpose seems most appropriate.
Acknowledgments
All photographs in this manuscripts are by Jack Kelley Clark, Principal Photographer, Cooperative Extension Service, University of California, Davis.
Literature Cited
Billiotti, E., and V.M. Stern etal . 1968. Report of the second session of the FAO panel of experts on integrated pest control. [Rome, Italy, September 19–24, 1968.] 48 p. Food and Agriculture Organization of the United Nations, Rome, Italy.
Brown, L.R. 1978. Insects of ornamental shrubs, shade trees and turf. p. 535–571. In : R.E. Pfadt (ed.). Fundamentals of applied entomology. 798 p. Macmillan Company, New York, N.Y.
Burnett, T. 1960. Control of insect pests. Proceedings of Federal American Society Experimental Biology 19:557–561.
California Department of Food and Agriculture. 1977. Estimated damage and crop loss caused by insect/mite pests: 1976. 24 p. California Department of Food and Agriculture Publication, Sacramento, Calif.
Doutt, R.L., and J. Nakata. 1973. The Rubus leafhopper and its egg parasitoid: an endemic biotic system useful in grape-pest management. Environmental Entomology 2(3): 381–386.
Eichlin, T.D. 1981. Location of gypsy moth finds in California—1981. p. 81–83. In : Cooperative plant pest report 4(8). California Department of Food and Agriculture, Sacramento, Calif.
Hoy, M. 1982. The gypsy moth—here again. California Agriculture 36(7):4–6.
Jensen, F.L., and D.L. Flaherty. 1981. Grape leafhopper. p. 98–110. In : Grape pest management. University of California Division of Agricultural Sciences Pub. 4105, Berkeley, Calif. 312 p.
Kahrl, W.L. (ed.). 1979. The California water atlas. Prepared by the Governor's Office of Planning and Research in cooperation with the California Department of Water Resources, State of California, Sacramento. 118 p.
Knipling, E.F. 1979. The basic principles of insect populations, suppression and management. US Department of Agriculture Handbook 512, Washington, D.C. 623 p.
Odum, E.P. 1971. Fundamentals of ecology. 574 p. Saunders, Philadelphia, Penn.
Purcell, A.H. 1981. Pierce's disease. p. 6269. In : Grape pest management. University of California Division of Agricultural Sciences Pub. 4105, Berkeley, Calif. 312 p.
Smith, R.F., and W.W. Allen. 1954. Insect control and the balance of nature. American Science 190:38–42.
Southwood, T.R.E. 1966. Ecological methods. 391 p. Chapman and Hall, London, England.
Stern, V.M., P.L. Adkisson, O. Beingolea, and G.A. Viktorov. 1976. Cultural controls. p. 593–613. In : Theory and practice of biological control. 788 p. Academic Press, Inc., New York, N.Y.
Stern, V.M., W.L. Peacock, and D.L. Flaherty. 1981. Western grapeleaf skeletonizer. p. 140–146. In : Grape pest management. University of California Division of Agricultural Sciences Pub. 4105, Berkeley, Calif. 312 p.
Stern, V.M., R.F. Smith, R. van den Bosch, and K.S. Hagen. 1959. The integrated control concept. In : The integration of chemical and biological control of the spotted alfalfa aphid. Hilgardia 29(2):81–154.
US Department of Agriculture. 1965. Losses in agriculture. US Department of Agriculture Handbook 291, Washington, D.C. 120 p.
van den Bosch, R., and A.D. Telford. 1964. Environmental modification and biological control. p. 459–488. In : P. DeBach (ed.). Biological control of insect pests and weeds. 844 p. Reinhold, New York, N.Y.
van Emden, H.F., and G.F. Williams. 1973. Insect stability and diverstiy in agroecosystems. Annual Review of Entomology 19:455–475.
Winkler, A.J., W.B. Hewitt, N.W. Frazier, and J.H. Frietag. 1949. Pierce's disease investigations. Hilgardia 19(7):207–264.