COLLECTION AND ANALYSIS OF BIOLOGICAL SAMPLES
Biological investigations in any area consist essentially of two parts: (1) descriptive and (2) analytical.
The descriptive part is concerned chiefly with determining the kind of the organisms present and their phylogenetic relationships, and with establishing their geographic and bathymetric distribution. This phase of marine biology was the first to develop historically, and is naturally the first in the investigation of new areas. The relative volume of strictly descriptive work gradually diminishes as the various groups of organisms and their distribution become known, but the application of the results will always be a part of biological studies.
The second, or analytical, phase is concerned above all with the factors operative in the development and distribution of the populations and with the biologic economy of the inhabitants as related to the inorganic and organic factors of the environment. Some of the factors operative in nature can be studied under controlled experiments in the field or in the laboratory, but in any event the information gained must be applied to an interpretation of the complex environmental conditions as they affect the lives of organisms found in the sea. Therefore, the collection of representative samples of plants and animals in nature and the analysis and interpretation of these samples become items of prime importance in order that true correlations can be found.
From the outset it must be realized that at best any sample of organisms collected from the sea is only a minute portion of a population that may occupy a tremendously large area of the sea or only a restricted portion thereof, and that the concentrations are often very irregular. It is only through the collection of numerous samples in various areas and seasons that a true picture of the members of the population—their distribution, life cycles, and interrelations—can be acquired, The nature, size, motility, and habitat of the organisms determine the type of collecting equipment to be used.
Collection of Benthic Organisms
In the intertidal region, only simple equipment is needed, but for collecting in deep water various types of dredges and grabs are used. A dredge (fig. 89b) consists essentially of a heavy rectangular or triangular iron frame to which is attached a baglike fish net of cotton or wire web to retain the organisms. The dredge is dragged on the bottom by means of a wire cable operated from a power winch aboard a slowly movingship. The size of dredges used varies greatly, depending upon the equipment available for their manipulation aboard ship. A practical size for use on a vessel 15 m or more in length and in water of moderate depth has a beam of about 1 m. Only stationary or slowly moving organisms are caught; microscopic forms are included if they are incidentally attached to larger animals and plants or in sediment that has not been completely washed through the meshes of the net during the ascent of the dredge. The
Illustrative of equipment for quantitative collecting of benthic life is the Petersen grab, or bottom sampler, developed by Petersen (1918) for quantitative investigation of benthic animals in relatively shallow waters. It consists of a pair of very heavy metal jaws that are held open during the descent (fig. 89a). When the grab strikes the bottom, the slackened cable releases the tension on a clutch that holds the jaws open, and, when the cable is again drawn tight by the winch aboard ship, the jaws snap shut by their own weight and enclose the material, including the sessile organisms, covering a measured area, usually 0.1 m2, of the bottom over which the open jaws descended. The organisms caught are screened from the bottom sediments, classified, and counted
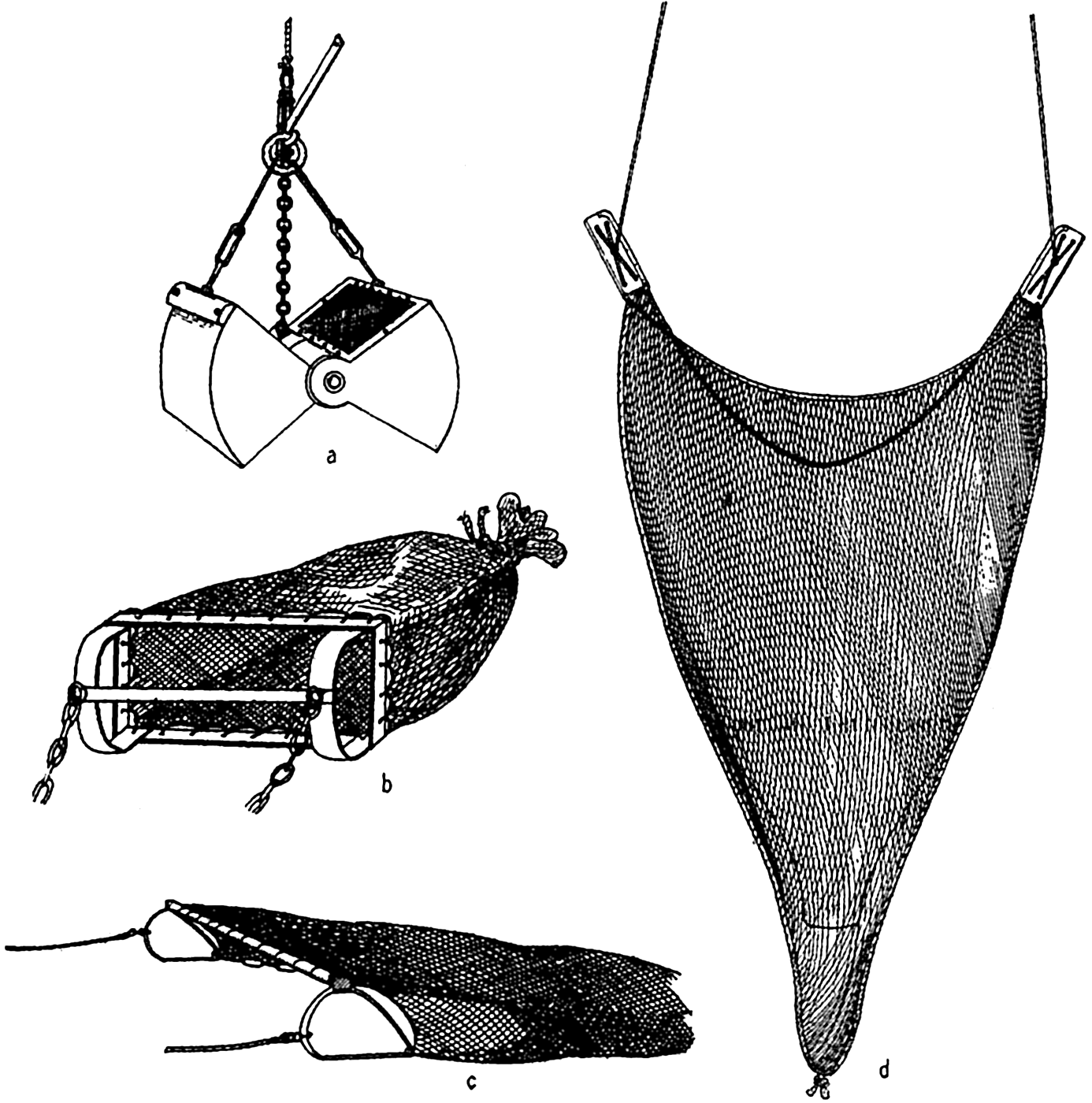
Gear for biological collecting: a, Petersen grab; b, dredge; c, beam-trawl; d, otter-trawl.
Small snappers and coring devices (figs. 84, 85) are useful in collecting quantitative biological samples only of microscopic organisms such as foraminifera and bacteria.
Collection of Nekton
In commercial fishing, diverse methods involving nets, trawls, traps, hooks, and harpoons are used, depending upon the animals sought, but we shall here consider only the trawl, which has been much used in collecting deep-water animals for scientific research. The beam trawl (fig. 89c) is constructed somewhat like the dredge, but the frame does not form digging and scraping edge and it may have a much larger opening, up to 15 m or more; since it is not designed to dig into the bottom, it may be towed at greater speed and thus catch the faster moving animals—shrimps, fishes, and so forth—that live on or near the bottom.
For pelagic trawling the otter trawl is used mostly. The opening to the web sack is kept distended, not by a rigid beam, as in the beam trawl, but by means of otter boards attached to opposite sides of the opening. Upon being towed, these boards are forced apart by the resistance of the water (fig. 89d). The span of the opening may be 20 to 26 m and the net may be 40 m long. A small otter trawl was employed successfully by the Michael Sars in fishing at depths as great as 5160 m.
The ring trawl is essentially a large, relatively coarse plankton net attached to a strong ring of large diameter and provided with a towing bridle (see below).
Collection of Plankton
A great variety of nets and other equipment has been used in obtaining samples of the phyto- and zooplankton. The plankton tow net was apparently first introduced by Johannes Müller in 1846 and has found the widest use of all plankton-collecting devices.
The plankton net consists of a filtering cone attached to a metal ring by which the net is towed through the water. Detailed instructions for cutting patterns for ordinary nets are given by Seiwell (1929). The filtering material forming the net is usually some grade of silk bolting cloth of the type used for sifting flour. It is numbered from 0000 to 25, depending upon the number of meshes per linear inch. The strands are so woven that the aperture size is not readily changed; hence they differ from those in ordinary weaving, where the strands cross each other alternately without a binding turn (fig. 90). The dimensions of the
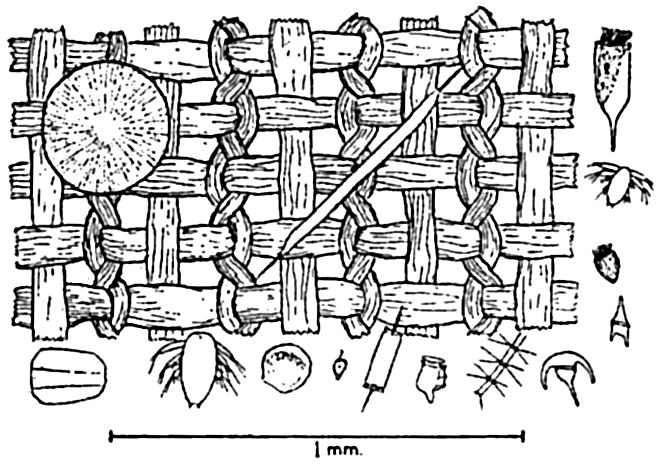
Enlarged camera lucida drawing to show weave of No. 20 bolting cloth, together with aperture size compared with the size of some common plankton organisms drawn to the same scale. Organisms, horizontal row: Coscinodiscus granii, nauplius Stage III Acartia; early clam larva, Prorocentrum micans Ditylum, Dinophysis, Chaetoceros, Ceratium tripos, Vertical row, down: Favella, nauplius Stage I, Acartia, Stenosomella, Ceratium furca. On cloth: Coscinodiscus wailsii and Rhizosolenia semispina, two unusually large diatoms.
At the cod, or tail, end of the net a detachable jar, vial, or small strainer of some type is placed to receive the concentrated tow.
A continuous plankton recorder has been developed by Hardy (1936) for towing behind a ship while under way at full speed. The machine is essentially a torpedo-shaped tube, circular or rectangular in cross section, and about 1 m long. The front end is provided with a small hole for entrance of water as the machine is hauled forward. This opening leads to a wider tunnel across which a long band of silk gauze is slowly wound from one spool to another and through which the water must pass before its exit by way of a hoIe at the rear end of the tube. The gauze, with the plankton screened from the water, is continuously rolled onto the storage spool. The winding is done by a propeller outside of the machine, and thus the speed at which the band is wound within the machine is controlled by the speed with which the whole machine is drawn through the water and is therefore in direct relation to the
| Silk No. | Meshes per inch | Size of aperture (mm) | Silk No. | Meshes per inch | Size of aperture (mm) |
|---|---|---|---|---|---|
| 0000 | 18 | 1.364 | 10 | 109 | 0.158 |
| 000 | 23 | 1.024 | 11 | 116 | 0.145 |
| 00 | 29 | 0.752 | 12 | 125 | 0.119 |
| 0 | 38 | 0.569 | 13 | 129 | 0.112 |
| 1 | 48 | 0.417 | 14 | 139 | 0.099 |
| 2 | 54 | 0.366 | 15 | 150 | 0.094 |
| 3 | 58 | 0.333 | 16 | 157 | 0.086 |
| 4 | 62 | 0.318 | 17 | 163 | 0.081 |
| 5 | 66 | 0.282 | 18 | 166 | 0.079 |
| 6 | 74 | 0.239 | 19 | 169 | 0.077 |
| 7 | 82 | 0.224 | 20 | 173 | 0.076 |
| 8 | 86 | 0.203 | 21 | 178 | 0.069 |
| 9 | 97 | 0.168 | 25 | 200 | 0.064 |
A smaller and simpler device known as the plankton indicator has been developed (Hardy, 1936) mainly for use by herring fishermen in determining the general type of plankton in the water before casting their nets. It consists of a tube 56 cm long, 8.9 cm in diameter, and with both ends tapering to 3.8-cm openings. Across the lumen of the tube a small disk of bolting cloth is placed for screening out the plankton as the tube is drawn forward through the water at full speed of the boat. The disk can be quickly removed and a clean one inserted, thus allowing frequent direct gross examinations to determine whether patches of phyto- or zooplankton are being traversed (see p. 907).
Zooplankton. Attempts to standardize the nets of various types have led to a comparison of the “catching power” of several common nets as compared to the Hensen egg net, which for basis of comparison is rated as having a catching power of 1.0 (Künne, 1933). In comparison with the Hensen net, the Nansen net was found to have a catching power of 0.87, or, in other words, under comparable conditions quantitatively it caught nearly the same number of animals in the same proportion as the Hensen net. The standard net, which is a modified Nansen net, caught much less, its catching power being only 0.1, while the Helgoland larvae net was rated at 4.1. It should be emphasized that the catching power refers only to the relative capacity of each net to catch the animals
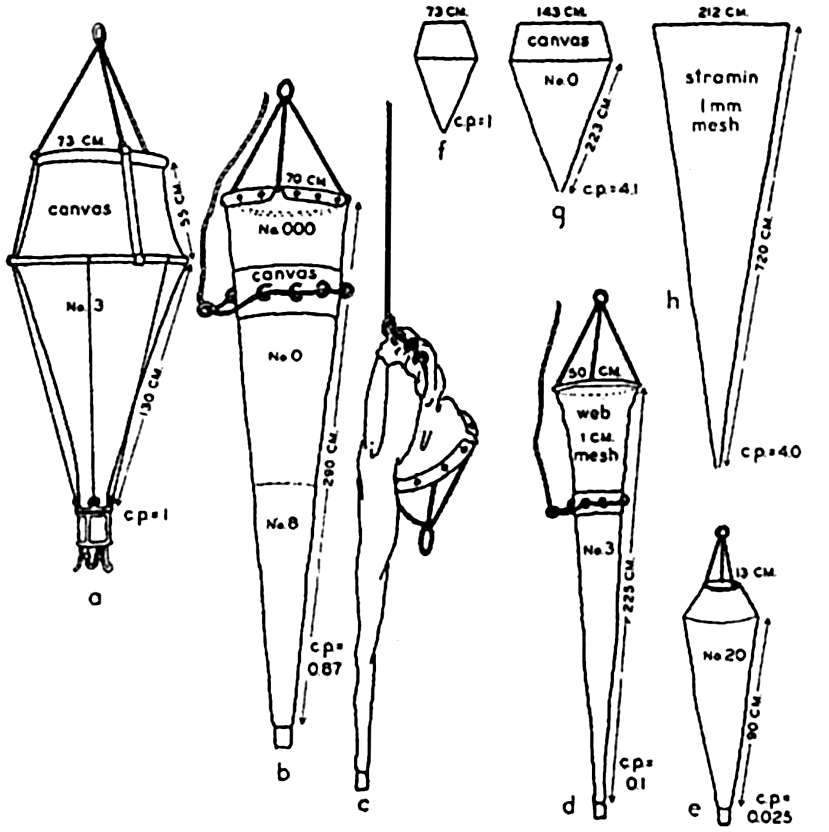
Types of plankton nets, their length, mouth diameter, type of filtering material, and relative catching power (c.p.), as explained in text: a to e drawn to same scale; f to h to a reduced scale. a, Hensen egg net; b, Nansen net open; c, Nansen net closed; d, standard net; e, medium Epstein net; f, Hensen egg net; g, Helgoland larva net; h, large vertical net of stramin.
The truncated nets, illustrated especially by the Hensen egg net and the Apstein net, have a reduced opening at the head end in order to increase the ratio of the filtering area of the net to its mouth area and at the same time, by means of the canvas head piece, to minimize backwash while the net is being towed through the water.
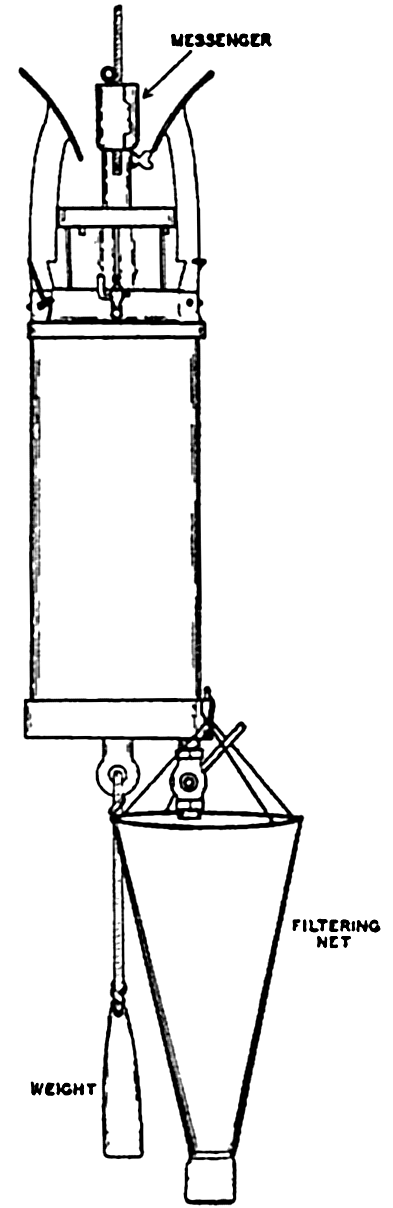
Some nets, illustrated by the Nansen and standard nets, may be closed by means of a messenger that activates a mechanism (fig. 92) releasing the bridle lines and thus causing the strain to fall upon the puckering line, which, at any desired depth, closes off the head end of the net (fig. 91b,c). Thus it is possible to obtain a sample of the plankton population at any subsurface level without contamination by the organisms living in the overlying water layers.
Single nets are sometimes hauled through the water horizontally at the desired depth, or a series of nets may be placed at given intervals on the wire in order to sample several water strata at once. Commonly a net is payed out to a given depth, and, after a period of fishing at this level, it is hoisted successively to a series of higher levels to fish for the same length of time at each before being hauled in, thus obliquely sampling several strata with one net. In the above methods of sampling, it is difficult to obtain an estimate of the amount of water that has been filtered. A more reliable estimate of the amount of water filtered is gained if the net is towed vertically instead of horizontally or obliquely.
In vertical hauls the net, with the cod end tied to a weight suspended by long lines from the top ring, is lowered to the desired depth and then raised vertically, filtering a column of water the length of the course traversed. There is always a considerable amount of backwash, however, resulting from the resistance of the net, especially if it is constructed of fine mesh or has become partially clogged by the plankton. Hence somewhat less water has been filtered than would have gone through an open ring of the diameter of the net mouth. Therefore, when using the area of the net opening as the cross section of the water column filtered, the figures obtained for the number of organisms caught are always minimal ones. As a rule, also, it is not possible to haul the net exactly vertically, since the boat from which it is operated usually drifts more or less, resulting in a haul somewhat longer than the true vertical. There appears to be no sure method of avoiding these types of collecting errors inherent in the use of ordinary nets. Sometimes a recording meter is placed in the mouth of the net so that the water passing into the net activates a small propeller attached to a counting mechanism. When properly calibrated, the number of revolutions of the propeller is taken to indicate the amount of water having passed through the net. From this the number of organisms per unit volume of water, usually per liter or per cubic meter, can be calculated, but, owing to unavoidable irregularities in filtration or analysis, the figures obtained are at best usually only
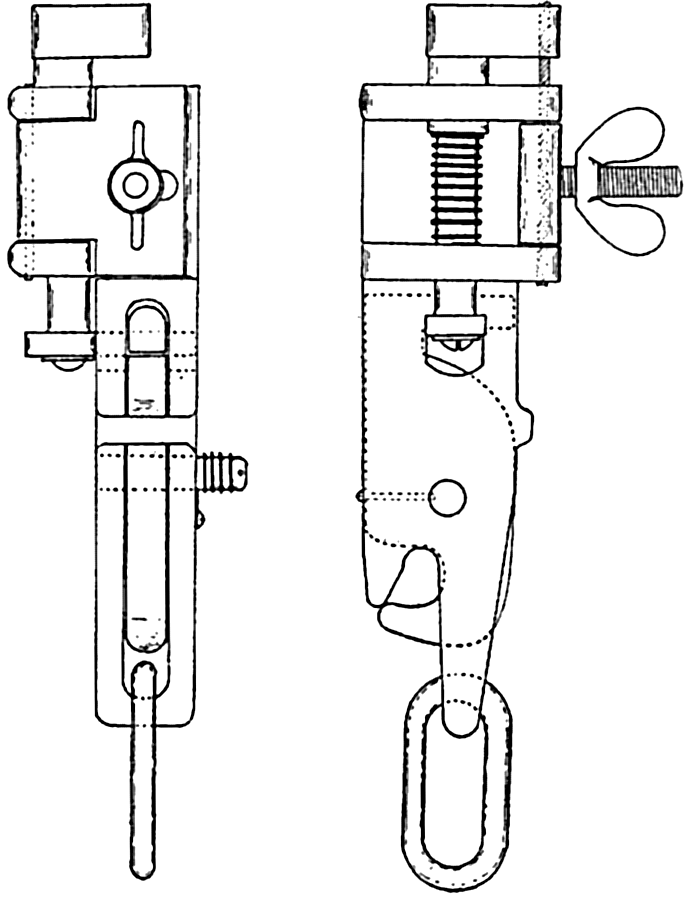
A tripping mechanism for closing nets and similar apparatus at subsurface levels; back and side views.
Total filtration and exact analysis, of course, will be more nearly realized in sparsely populated waters, while in dense populations or in the presence of gelatinous objects, such as jellyfishes or cast-off appendicularian vestments (“houses”), the errors must become greater. Coarse-meshed nets provided with a water-measuring device represent the most ideal combination for collecting, but they are highly selective and can be used only where such selection is desirable on the basis of size—for example, in the study of large fish eggs or larvae. For a statistical study of the variations in catch of plankton nets the reader is referred to Winsor and Clarke (1940), and the relevant works included in their bibliography.
In an attempt to overcome the uncertainties inherent in net collecting of plankton, various devices (bottles and buckets) have been designed to entrap a definite amount of water together with its plankton population from a definitely known depth. Pumps operated from aboard ship and provided with a long hose, the intake of which can be lowered to the desired depth, have been used successfully, especially in fresh water, for sampling the population at depths down to about 75 m (Birge and Juday, 1922). Known amounts of water from a series of depths can in this way be filtered through fine nets, as the water is delivered from the pump, which is also provided with a water meter. These methods are excellent for some purposes, but are also subject to grave limitations, since they are useful only for inactive or relatively slowly moving organisms, when abundant and rather evenly distributed, such as diatoms, dinoflagellates, protozoa, and sometimes larvae. Fast moving, sparse, and irregularly distributed forms are not likely to be caught with sufficient regularity to give significant data. The ease with which such samples can be taken makes more samplings possible, and this advantage overcomes somewhat the adverse features.
Except for special studies, the zooplankton caught must be preserved in the field. Usually a 4 per cent solution of formaldehyde (preferably neutral) is used for this purpose. The collecting data on the label, placed inside the jar, should include serial number, date, hour, station number, depth sampled, and type of net used and how operated. The laboratory analysis of the samples usually consists of identifying and counting all of the desired species occurring in an aliquot portion of the well-mixed sample. From this is computed the total number of the selected species occurring in the whole sample. Finally, the volume of water filtered by the net in taking the sample having been approximately determined, the population is reported as the number of different
Allen phytoplankton bottle with filtering net in place for emptying.
Phytoplankton. The regular Nansen hydrographic water bottle (fig. 87) is much used for collecting phytoplankton samples, or, if larger samples are desired, the Allen bottle (fig. 93), with a capacity of 5 l, can be used. When the Nansen bottle is employed, samples from certain levels, usually 1, 10, 25, 40, 75, 150 m or more, are tapped off directly into as many citrate bottles, neutral formaldehyde preservative is added, and the bottles are stored for examination in the laboratory. When the collection has been made with an Allen bottle (or similar large collector) at similar depths, the catch is immediately concentrated in a small volume of water (100–150 ml) by filtering through a small net of No. 25 bolting cloth. The sample is then preserved as above for study in the laboratory.
When the sample has not been concentrated in the field, the laboratory analysis consists first of centrifuging a measured portion (say 10 to 50 ml, depending upon the abundance of plankton) of the well-shaken sample. The clear fluid is withdrawn by means of a pipette, and the organisms that have been thrown down in the centrifuge tube are removed, together with the remaining fluid, to a cross-ruled glass slide. They are then covered by a cover slip, and the organisms are identified and enumerated under a compound microscope (Gran, 1932). The numbers obtained form tbe basis for calculating and reporting the
If the sample was concentrated in the field by filtering, an aliquot portion of the concentrated sample is placed in a Sedgwick-Rafter counting cell holding 1.0 ml of the sample, and the organisms are examined and reported as above.
The phytoplankton population may also be conveniently analyzed chemically by extracting the yellow pigments with acetone and reporting the population in numbers of plant-pigment units. In this analysis, the diatoms are filtered from a known quantity of water, and the pigments are then extracted with a measured volume of acetone. The tinted acetone resulting is compared colorimetrically with an arbitrary standard prepared by dissolving 25 mg of potassium chromate and 430 mg of nickel sulphate in 1 l of water. One milliliter of the standard solution is equivalent to one “pigment unit.” For a fuller discussion of this method and of other means of estimating phytoplankton production through utilization of plant nutrients and through oxygen production and consumption, see chapter XIX.
Bacteria. The collection of bacterial samples presents a very special problem, since they must be sterilely taken. In fig. 94 is shown a collecting bottle in the sampling frame with a combination glass and rubber filling tube. This tube is so designed that when the glass section is broken, the rubber part, which is under tension, projects the distal end of the tube, with the intake orifice, at some distance from the bottle and other equipment (ZoBell, 1941). The sea water entering the sterile bottle is thus free from contamination by bacteria that might otherwise be forced in from the surface of the sampling equipment. The frame, with the sampling bottle in place, is attached to the cable, and, at the desired depth, a messenger trips the tube-breaking device. Since the bottles are lowered empty, the depth at which samples can be taken by this means is limited to water pressures that will not break the bottle or force in the stopper. Experiments using rubber bottles to overcome these difficulties are now in progress. Laboratory analysis of the sample collected consists of plating out a known portion of the sample on nutrient material and noting the maximum number of colonies that grow, each
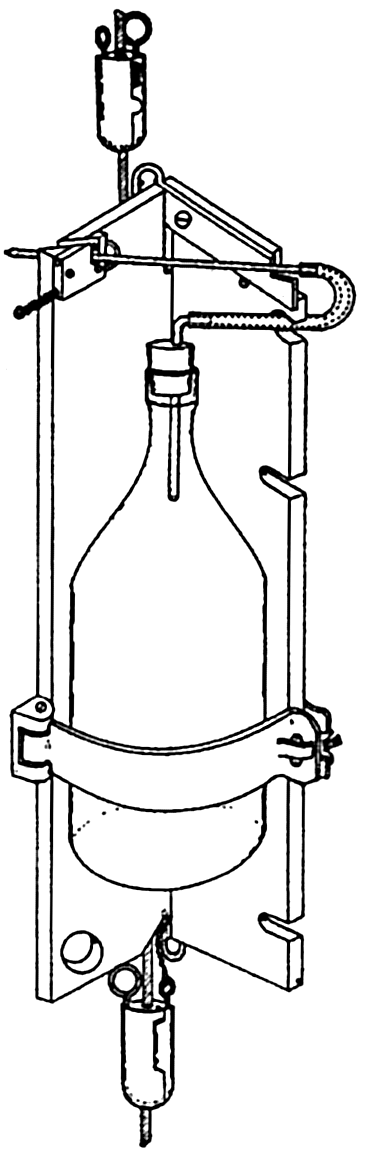
Bacteria sampling bottle in frame attached to cable. The upper messenger breaks the glass intake tube and releases the second messenger when samplers are used in series.
The bacterial population in collected water or mud samples changes very rapidly, making it necessary to plate out the samples as soon as they are obtained in the field in order to obtain the most reliable estimates of the numbers of bacteria in situ. The type of culture medium used is of vital importance in obtaining a growth response from the maximum number of viable bacteria in the sample. Only media made up with sea water are effective (ZoBell, 1941).
The bacteria from bottom sediments are obtained by sterilely removing a sample of the undisturbed material in the center of a core taken with some type of coring device (p. 345).
Interpretation of Plankton Observations
In the interpretation of field observations on plankton, it must be remembered that the different requirements of separate species lead to a more or less complete change of the elements in the population when external factors, especially temperature and nutrients, become altered in the water mass inhabited. When such a biological change takes place within a rather well-defined water mass, whether moving or stationary, we may speak of it as an individual population succession. This must not be confused with a change of population resulting from a sequence of distinct water masses flowing with their distinct populations into a given geographical position where successive series of observations are being made. This type of change may be termed a local sequence. It is frequently not possible to distinguish between these two important types of changes that may occur in the population of an area under investigation, though hydrographic data accompanying biological sampling will aid materially in the interpretation of the biological data by providing information on the nature of residual movements of water. Local sequences in populations are likely to be more sudden than individual population successions, since the latter depend upon biological development rather than upon a simple physical shift of water masses. The former may also at times be slow when neighboring populations become mixed or scattered only through the process of advection or lateral mixing.
An example embracing both local sequence and individual population succession in a population is illustrated by the recent observations of Redfield (1939) on the history of populations of the pteropod Limacina retroversa, its entrance and sojourn in the Gulf of Maine (see also p. 864). A population of small individuals appears in the Gulf in December with inflowing water from the east. Caught in the cyclonic circulation of the Gulf, they gradually decrease in number through mortality or some are
The changes occurring in a population may involve a succession of development stages of a given species. In view of this it should be noted that any biological succession observed may result from two causes. There may be (1) a change in composition of species, owing to different biological responses to physical or chemical changes (that is, rise or fall in temperature or nutrient state of the water) that have occurred within the individual water mass, or (2) a change in the relative maturity of the population, owing simply to the passage of time and to chronologically developed stages in the life history of the individuals of one or more species. A change in the phytoplankton involving a succession of species is well illustrated in boreal water, where in late spring or summer there is commonly a drop in the concentration of diatoms in a predominantly diatom plankton and an accompanying or following increase in dinoflagellates. Here two factors, in particular, are operative in the individual water mass: (1) an increase in temperature (owing to advance of the season), which favors the warmth-loving dinoflagellates, and (2) depletion of plant nutrients by the diatoms to a point suboptimal for their abundant proliferation, but still sufficient for the dinoflagellates, which are able to reduce the nutrients further and through their motility to adjust themselves in some degree with respect to favorable light conditions (see p. 765). The phosphate and nitrate requirements of certain dinoflagellates (Ceratium sp., Peridinium sp., Prorocentrum micans) have been found experimentally to be exceedingly low (Barker, 1935). The maximum rate of division has already been reached with 0.1 parts per million of nitrogen, and this element is probably a limiting factor only at dilutions of 0.01 to 0.001 parts per million.
A biological succession involving the percentage composition of developmental stages of a single dominant species associated with lapse of time is illustrated in studies of the life cycle of Calanus finmarchicus in the relatively slowly flushed waters of the Clyde Sea area (p. 323) where in Loch Striven it was possible (Marshall, Nicholls, and Orr, 1934) to trace the successive developmental stages and broods of Calanus that occur during the seasons.