10.2—
Polysomes
10.2.1—
Structure of Ribosomes
Ribosomes are classified as eukaryotic or prokaryotic in type, on the basis of: (a) sensitivity to various antibiotics; (b) functional interchangeability of soluble factors and ribosomes from different sources; and (c) structure and sedimentation characteristics, e.g. 70s (prokaryotic) or 80s (eukaryotic).
Bacterial ribosomes, plant chloroplast and mitochondrial ribosomes are classified as prokaryotic, even though they may not always exhibit all of the above characteristics, e.g. plant mitochondrial ribosomes have sedimentation values of about 80s.
Plant and animal cytoplasmic ribosomes are of the eukaryotic type and have sedimentation values of about 80s. This class of ribosomes are heterogeneous in size however, with most of the differences accounted for by differences in the large subunit, the small subunit having been conserved during evolution (Cammarano et al., 1972a,b & c).
Measurements of the size of plant cytoplasmic ribosomes in the electron microscope vary, but they are approximately 25 × 20 nm. Miller et al., (1966) have described them as being acorn-shaped, and several workers have described a cleft in the small subunit. In the rat liver ribosome model of Nonomura et al., (1971) there is a tunnel between the two subunits, directly under the cleft in the small subunit, which is thought to accommodate the mRNA. Ribosomes require Mg2+ for structural integrity and dissociate at low Mg2+ concentrations into a large and a small subunit with sedimentation values of about 60s and 40s respectively (Ajtkhozhin et al., 1972). There is good evidence that mRNA attaches to the small subunit before the addition of the large subunit completes the ribosome structure, and the different roles of the two subunits are a feature of all the models of ribosome structure and function (see Noll et al., 1973). The 80s ribosome of plants corresponds to a molecular weight of 3.9 × 106 daltons, with molecular weights for the large subunit of 2.4 × 106 daltons and the small subunits, 1.5 × 106 daltons; the small subunit is of the same molecular weight as its mammalian counterpart, the large subunit is smaller. The large subunit of plant ribosomes contains one molecule of 25s RNA, hydrogenbonded to 1 molecule of 5.8s RNA (Payne & Dyer, 1972), and 1 molecule of 5s RNA; the small subunit contains 1 molecule of 18s RNA. The molecular weight of the 25s RNA is 1.3 × 106 daltons, and that of the 18s is 0.7 × 106 daltons (Loening, 1968). The large subunit contains 46% protein, and the small 54%.
The large subunit of pea seedling ribosomes contains 44–45 proteins and the small subunit 32–40 proteins, of which most are basic and of molecular weights between 20 × 103 and 30 × 103 daltons. Some proteins may be represented by more than one copy per ribosome. Proteins extracted from cytoplasmic
and chloroplast ribosomes of the same species, show little similarity in two-dimensional electrophoresis or by immunological comparison and are significantly less similar than are the cytoplasmic ribosomes of different species, e.g. beans and wheat (Gualerzi et al., 1974).
Before a complete understanding of the mechanism of protein synthesis is elucidated, it will be necessary to know the spatial relationships and three-dimensional structures of the proteins and the RNAs of the ribosome, as well as those of the associated molecules which also play a part in protein synthesis. Information on the structure of the proteins and the RNA molecules of the E. coli ribosome, together with reconstitution experiments, is now well advanced (Traub & Nomura, 1968; Nashimoto et al., 1971; Wittmann, 1973; Anderson et al., 1974; Nierhaus & Dohme, 1974).
10.2.2—
Free and Membrane-Bound Polysomes
Ribosomes associate with mRNA to form polysomes, as can be seen in the electron micrograph (Fig. 10.1); the size of the polysome varies according to the length of the mRNA and the number of attached ribosomes. Polysomes are found either free in the cytoplasm or attached to the surface of membranes of the endoplasmic reticulum (ER) and the nucleus. In animal embryonic cells, most of the polysomes are free and engaged in protein synthesis for internal use, whereas in those differentiated animal cells from which large amounts of protein are exported, most of the polysomes are found attached to the ER. Thus, the generalization arose that membrane-bound ribosomes synthesize protein for export and free ribosomes for intracellular use. It soon became clear however, that membrane-bound ribosomes occur in some tissues which do not export protein, and that in cells where all the protein synthesized is for internal use, different classes of protein are synthesized on the two types of ribosomes. Less information is available for plants. In the developing broadbean seed the highly vacuolate, relatively membrane-free cells of the cotyledon are transformed just prior to the onset of storage protein synthesis, into cells whose cytoplasm is vesicular and in which ER with attached ribosomes is very prominent. This new protein synthesis machinery is assembled at a precise time in the course of seed development for the production, in large amounts, of the storage proteins of the seed (Bailey et al., 1970; see Fig. 10.2). Later, during dehydration of the seed, ribosomes become detached and the population of free ribosomes thereby increased. A similar series of events has been recorded for the developing seeds in many other plants.
The way in which ribosomes attach to membranes is not clear; the ribosomes themselves, the membranes and the protein being synthesized, have all been suggested as being involved in the binding. There is evidence from animals that the large subunit is in close contact with the membranes, and Baglioni et al. (1971) have postulated that the large subunit binds directly to the membrane, probably at a specific binding site (Sunshine et al., 1971), and that a protein on
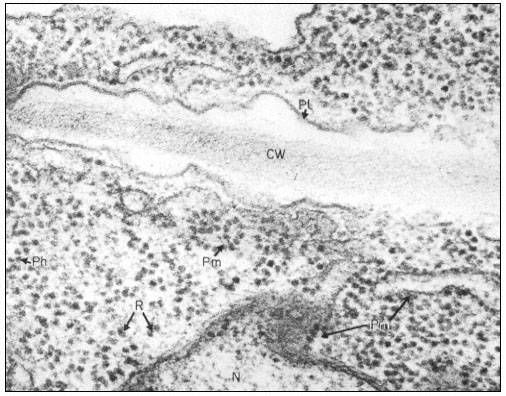
Figure 10.1
Electron micrograph of a thin section of parts of two adjacent cells in a
shoot apex of pea showing ribosomes and polyribosomes; CW = Cell Wall, Pl
= Plasmalemma, N = Nucleus, Pm = Membrane bound polysomes, R = Ribosome
Ph = Polysome helix. By courtesy of A. D. Greenwood, Department of Botany
and Plant Technology, Imperial College of Science and Technology, London.
the membranes is responsible for the attachment (James et al., 1969). Alternatively, it has been suggested that one of the proteins of the large subunit is responsible, whereas other observations suggest that the binding may be dependent on the nascent polypeptide chain. It now seems likelly that, in vivo, membrane-bound ribosomes synthesize a different class of protein to free ribosomes, whereas in vitro, proteins of both classes are synthesized by both types of ribosomes, suggesting the control is not a function of the ribosome itself.
10.2.3—
Isolation and Purification
Ribosomes and polysomes are normally isolated from plants and purified by sedimentation through sucrose cushions as originally described by Wettstein
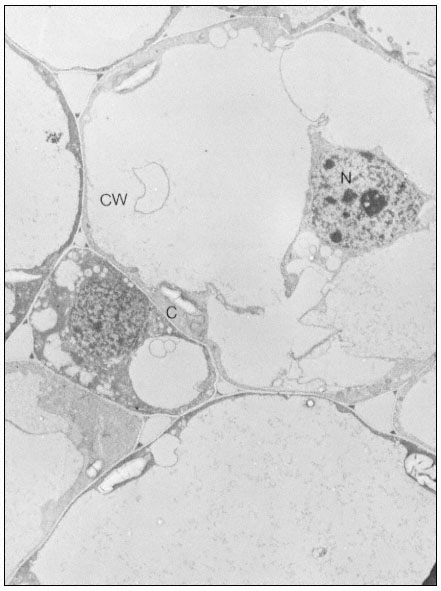
Figure 10.2
Electron micrographs of developing seeds of Vicia faba.
(a) 25 days after fertilization. Ribosomes free in the cytoplasm. Very little ER present.
et al. (1963). In a typical procedure, the material is homogenized in 0.25 M sucrose containing 200 mM -Tris-HCl, pH 8.5 at 2ºC, 500 mM -KCl and 15 mM -MgC12 , with a Willems Polytron for 3 seconds at a speed setting of 8. The homo-
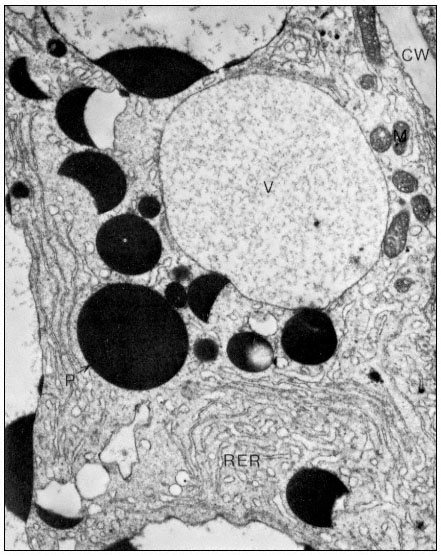
Figure10.2
(b) 55 days after fertilization, showing most of the polysomes bound to ER and
storage protein being laid down. C = Cytoplasm, P = Protein body, CW = Cell Wall,
RER = Endoplasm reticulum with polysomes attached, N = Nucleus, V = Vacuole.
From Boulter et al. (Qual. Plant. XXIII, 239–50, 1973).
genate is filtered through Miracloth and Triton X-100 is added to a final concentration of 2% (v/v). The filtrate is centrifuged at 104 × g for 10 min at 2°C and the ribosomes and polysomes are recovered from the supernatant by layering over a 3 ml cushion of 0.1 M sucrose in 50 mM -Tris-HCI, pH 8.5, 50 mM -KCl
and 10 mM -MgCl2 , followed by centrifugation for at least 44 x 107 g-min, as described by Leaver & Dyer (1974) (Fig. 10.3). The inclusion of the detergent
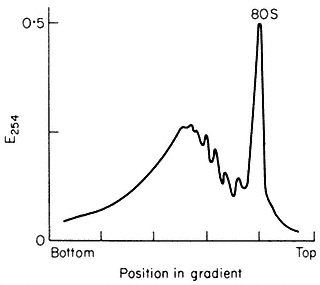
Figure 10.3
Sucrose density profiles of ribosomes and polysomes from Pisum
sativum. Ribosomes recovered after 6 h. centrifugation through
1 M -sucrose cushion. E254 = absorbance at 254 nm
(From Leaver & Dyer Biochem. J. 144, 165–7, 1974.)
Triton X-100 is necessary since plant materials contain a proportion of polysomes which are membrane-bound and the addition of Triton X-100 solubilizes the endoplasmic reticulum, so releasing them. The method gives a preparation of both polysomes and ribosomes and the latter can be removed by centrifugation techniques or, alternatively, the whole preparation can be converted to ribosomes by exposure of the plants to nitrogen gas for at least 1 hour prior to extraction. Since different plants and even different tissues from the same plant contain different amounts of membrane-bound to free polysomes and different free polysomes to free monoribosome ratios, isolation conditions may need to be varied for optimum results with different experimental materials. The situation is further complicated by the fact that plant mitochondrial ribosomes, although not functionally of the 80s type, sediment at 80s (Leaver & Harmy, 1973), and can contaminate cytoplasmic preparations to varying extents, depending on the experimental material. Damage to ribosomes, both structural and by the removal of associated proteins, can occur during isolation and preparation. The extent of this damage can be assessed, to some extent, by extracting the RNA and fractionating it by polyacrylamide gel electrophoresis (see Leaver & Key, 1970).